Synthesis method of cytochalasin compound and cytochalasin derivative
A technology for cytochalasin and a synthesis method, which is applied in the field of synthesis of cytochalasin compounds and cytochalasin derivatives, can solve the problems of high toxicity, high price and low yield of reagents, and achieves safe reagents and simple operation. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
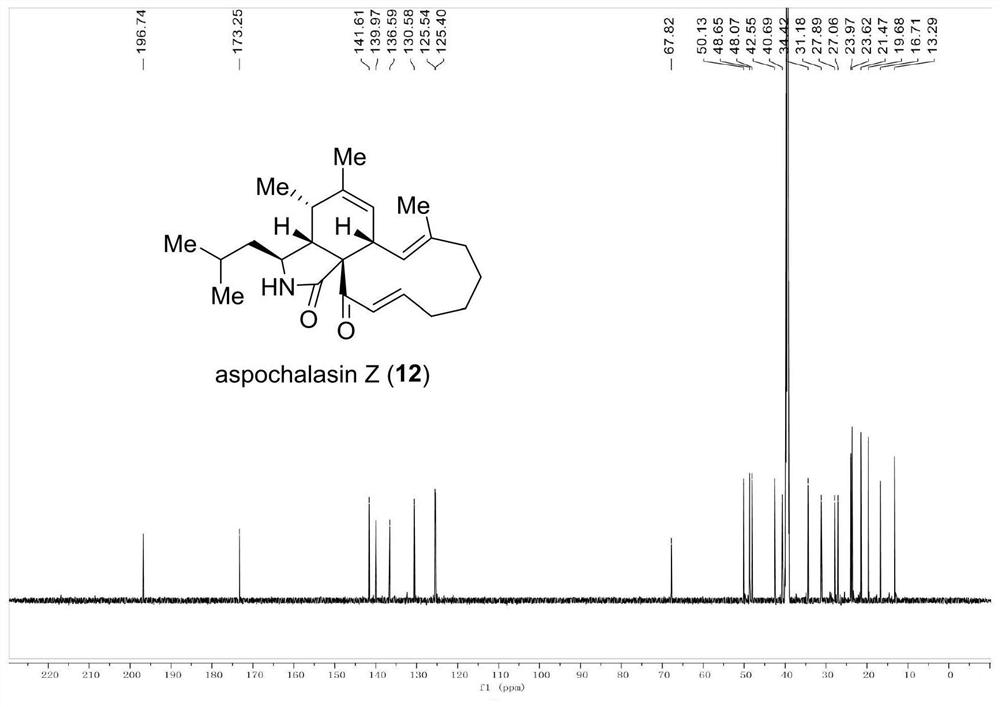
[0091] Embodiment 1 of the present invention discloses a preparation method of cytochalasin compound aspochalasin Z, comprising the following steps:
[0092]
[0093] (A) Dissolve 1.6mmol of compound A in 1.6L of anhydrous dichloromethane, then add 3.23mmol of 1,5,7-triazabicyclo[4.4.0]-5-decene and stir at room temperature for 14h, Then add saturated aqueous sodium bicarbonate solution to quench the reaction;
[0094] (B) Take the above-mentioned quenched reaction product and add ethyl acetate to extract, then wash with saturated aqueous sodium chloride solution, then add anhydrous sodium sulfate to dry, filter, concentrate under vacuum, and finally obtain after purification by silica gel column chromatography The cytochalasin compound aspochalasin Z (293 mg, yield 49%, theoretical yield).
[0095] The theoretical yield of the cytochalasin compound aspochalasin Z was calculated to be 593 mg; the actual yield was 293 mg when measured on the obtained product; the yield calc...
Embodiment 2
[0107] Embodiment 2 of the present invention discloses a preparation method of cytochalasin compound aspochalasin Z, comprising the following steps:
[0108] (A) Dissolve 1.6mmol of Compound A in 32mL of anhydrous dichloromethane, then add 3.21mmol of 1,5,7-triazabicyclo[4.4.0]-5-decene and stir at room temperature for 14h, then Add saturated aqueous sodium bicarbonate solution to quench the reaction;
[0109] (B) Take the above-mentioned quenched reaction product and add ethyl acetate to extract, then wash with saturated aqueous sodium chloride solution, then add anhydrous sodium sulfate to dry, filter, concentrate under vacuum, and finally obtain after purification by silica gel column chromatography The cytochalasin compound aspochalasin Z (223mg, yield rate 38%, theoretical yield 593mg).
[0110] The theoretical yield of the cytochalasin compound aspochalasin Z was calculated to be 593 mg; the actual yield was 223 mg when measured on the obtained product; the yield calcul...
Embodiment 3
[0121] Embodiment 3 of the present invention discloses a preparation method of cytochalasin compound aspochalasin Z, comprising the following steps:
[0122] (A) Dissolve 1.6mmol of compound A in 1.6L of anhydrous dichloromethane, then add 4.81mmol of 1,5,7-triazabicyclo[4.4.0]-5-decene and stir for 14h at room temperature. Then add saturated aqueous sodium bicarbonate solution to quench the reaction;
[0123] (B) Take the above-mentioned quenched reaction product and add ethyl acetate to extract, then wash with saturated aqueous sodium chloride solution, then add anhydrous sodium sulfate to dry, filter, concentrate under vacuum, and finally obtain after purification by silica gel column chromatography The cytochalasin compound aspochalasin Z (285 mg, yield 48%, theoretical yield 593 mg).
[0124] The theoretical yield of the cytochalasin compound aspochalasin Z was calculated to be 593 mg; the actual yield was 285 mg when measured on the obtained product; the yield calculate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com