Preparation method of key intermediate of JAK3 enzyme inhibitor
A technology of inhibitors and intermediates, applied in the field of medicine, can solve the problems of high cost, low yield, unsuitable for large-scale production, etc., and achieve the effects of mild reaction conditions, high reaction controllability, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention is specifically described below by the examples, it is necessary to point out again that the following examples are only used to further illustrate the present invention, and cannot be interpreted as limiting the protection scope of the present invention
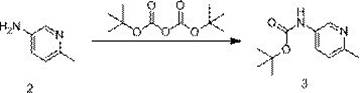
[0033] Synthesis of (6-methylpyridin-3-yl) tert-butyl carbamate (3)
[0034]
[0035] In a 1000ml three-necked flask, add 6-picoline-3-amine (80.00g, 739.75mmol, 1.0eq), ethanol (320ml), cool down to 0°C and stir. Add slowly (Boc) 2 O (209.89g, 209.89mol, 1.3eq), added dropwise completely, transferred to room temperature and stirred overnight. TLC monitoring (PE / EA=3:1) showed that the reaction was complete, filtered under reduced pressure, washed the filter cake with a small amount of absolute ethanol, and concentrated the filtrate under reduced pressure to obtain a crude oily product.
[0036] Transfer the crude product to a 2000ml three-necked flask, add ethyl acetate (160ml) and n-hexane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com