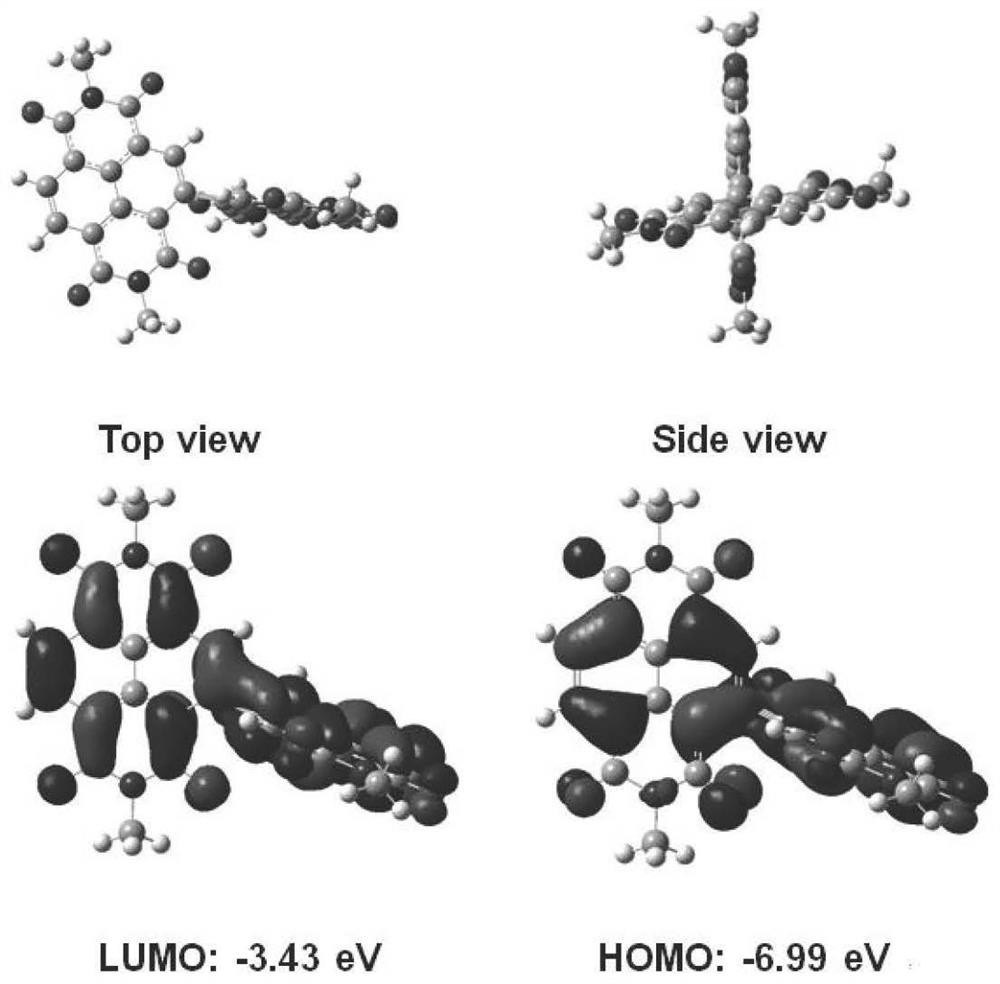
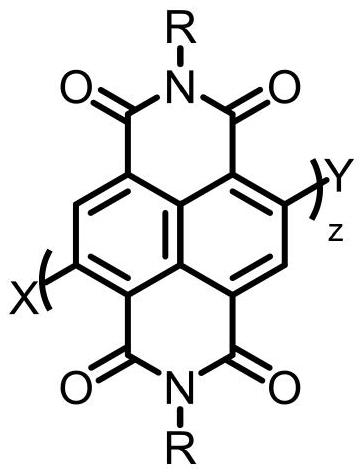
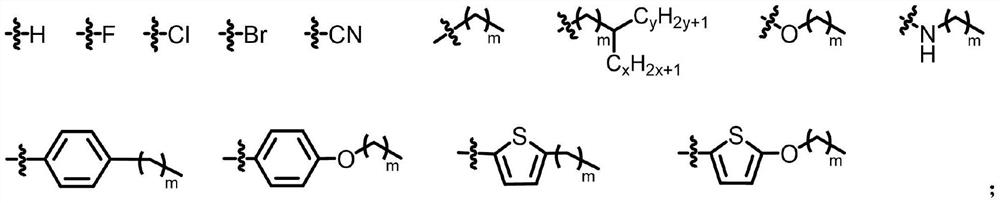
Small organic molecule electron transport materials based on naphthalimide units and their applications
An electron transport material, the technology of naphthalene diimide, which is applied in the field of organic small molecule electron transport materials, can solve the problems of insufficient device efficiency and stability, high LUMO energy level, unfavorable electron injection and transport in devices, etc., to achieve Improve electroluminescent efficiency, improve stability, and facilitate processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: DNDI (4-b-C8) organic small molecule, the structural formula is as follows:
[0048]
[0049] The preparation method is as follows: take 1,4,5,8-naphthalenetetracarboxylic anhydride (2.68g, 10mmol); 25mL of concentrated sulfuric acid in a 100mL single-neck flask, stir for 1h until the system is clear, add brominated reagent 1,3 in batches, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol), react at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol, and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to give white crystals of brominated naphthalene tetracarboxylic anhydride (Br-NDA). Yield 2.42 g, 70% yield.
[0050] NMR analysis of the prepared monomers, the results are as follows:1 H NMR (400MHz, DMSO-d 6 ): δ8.71(s, 1H), 8.57(d, 1H), 8.21(d, 1H); 13 C NMR (100MHz, DMSO-d 6 ): δ168....
Embodiment 2
[0059] Embodiment 2: DNDI (4-d-C5) organic small molecule, the structural formula is as follows:
[0060]
[0061] The preparation method is as follows: take 1,4,5,8-naphthalenetetracarboxylic anhydride (2.68g, 10mmol); 25mL of concentrated sulfuric acid in a 100mL single-neck flask, stir for 1h until the system is clear, add brominated reagent 1,3 in batches, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol), react at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol, and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to give white crystals of brominated naphthalene tetracarboxylic anhydride (Br-NDA). Yield 2.42 g, 70% yield.
[0062] NMR analysis of the prepared monomers, the results are as follows: 1 H NMR (400MHz, DMSO-d 6 ): δ8.71(s, 1H), 8.57(d, 1H), 8.21(d, 1H); 13 C NMR (100MHz, DMSO-d 6 ): δ16...
Embodiment 3
[0071] Embodiment 3: DNDI (4-n-C6) organic small molecule, the structural formula is as follows:
[0072]
[0073] The preparation method is as follows: take 1,4,5,8-naphthalenetetracarboxylic anhydride (2.68g, 10mmol); 25mL of concentrated sulfuric acid in a 100mL single-neck flask, stir for 1h until the system is clear, add brominated reagent 1,3 in batches, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol), react at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol, and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to give white crystals of brominated naphthalene tetracarboxylic anhydride (Br-NDA). Yield 2.42 g, 70% yield.
[0074] NMR analysis of the prepared monomers, the results are as follows: 1 H NMR (400MHz, DMSO-d 6 ): δ8.71(s, 1H), 8.57(d, 1H), 8.21(d, 1H); 13 C NMR (100MHz, DMSO-d 6 ): δ16...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com