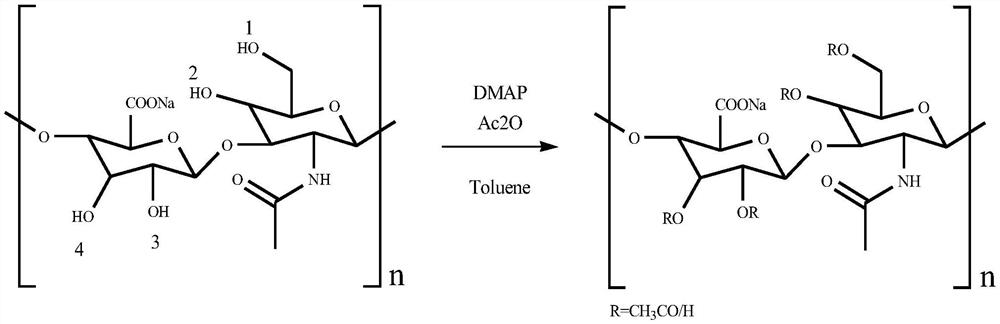
Preparation method of acetylated sodium hyaluronate
A technology of sodium hyaluronate and hyaluronate, which is applied in the field of organic chemical synthesis, can solve problems such as peroxide decolorization safety hazards, excessive iron and chromium content in products, and reduced degree of acetyl substitution, etc., to achieve mild reaction and state Good, the effect of reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of acetylated sodium hyaluronate in this embodiment comprises the following steps:
[0024] (1) Under the protection of nitrogen, weigh 50.00g of HA with a molecular weight of 10KDa in a 1000mL reaction bottle, add anhydrous solvent 250mL toluene, heat up to 60°C and stir until it is completely dissolved and becomes a homogeneous phase, then add molecular sieves to absorb water in the reaction ; After that, cool down the reaction system to -5°C, add 50mL of acetic anhydride dropwise to the reaction solution, then dissolve 0.25g DMAP with 4mL of toluene solution and add dropwise to the reaction solution, react at room temperature for 1 hour, then heat to 40°C for reaction 4h obtained light yellow turbid solution;
[0025] (2) adding an equal volume of water to quench the remaining acetic anhydride, and filtering the reaction solution to remove molecular sieves;
[0026] (3) The reaction solution was purified by membrane filtration, and a small amo...
Embodiment 2
[0033] The preparation method of acetylated sodium hyaluronate in this embodiment comprises the following steps:
[0034] (1) Under nitrogen protection, weigh 30.00g of HA with a molecular weight of 1000KDa in a 1000mL reaction bottle, add anhydrous solvent 300mL toluene and heat up to 70°C and stir (the temperature rise is to dissolve sodium hyaluronate, the larger the molecular weight, the more insoluble ), until the complete solution becomes homogeneous, adding molecular sieves to absorb water in the reaction; then cooling the reaction system to 0°C, adding 90mL acetic anhydride dropwise to the reaction solution, and then dissolving 0.2g DMAP with 4mL toluene and adding dropwise to In the reaction solution, react at room temperature for 1 hour, then heat at 50°C for 12 hours to obtain a light yellow turbid solution;
[0035] (2) adding an equal volume of water to quench the remaining acetic anhydride, and filtering the reaction solution to remove molecular sieves;
[0036]...
Embodiment 3
[0041] The preparation method of acetylated sodium hyaluronate in this embodiment comprises the following steps:
[0042] (1) Under the protection of nitrogen, weigh 20.00g of HA with a molecular weight of 200KDa in a 1000mL reaction bottle, add anhydrous solvent 400mL toluene, heat up to 70°C and stir until it is completely dissolved and becomes a homogeneous phase, then add molecular sieves to absorb water in the reaction ; Afterwards, the temperature of the reaction system was lowered to -5°C, 100mL of acetic anhydride was added dropwise to the reaction solution, and then 0.24g DMAP was dissolved in 4mL of toluene and added dropwise to the reaction solution, reacted at room temperature for 1h, and then heated at 70°C for 48h to obtain Pale yellow turbid solution;
[0043] (2) adding an equal volume of water to quench the remaining acetic anhydride, and filtering the reaction solution to remove molecular sieves;
[0044] (3) The reaction solution was purified by membrane fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com