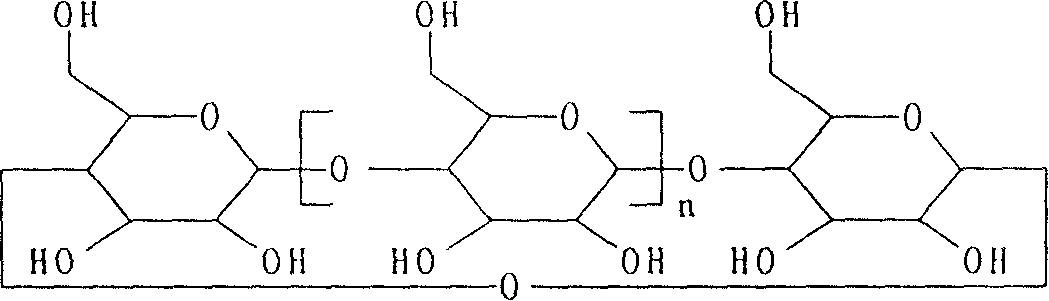
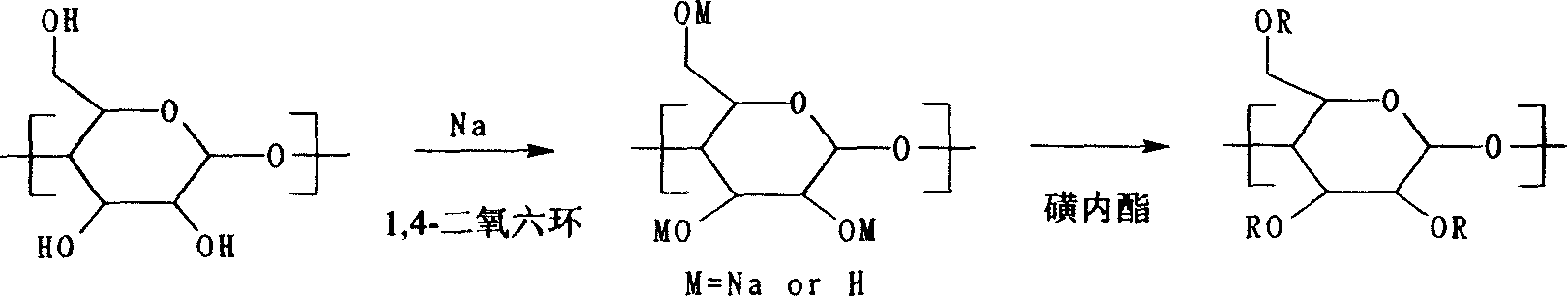
Synthetic process for water soluble sulfoalkyl ether-beta-cyclic dextrine
A synthesis process and sulfoalkyl ether technology are applied in the field of new synthesis process of water-soluble sulfoalkyl ether-β-cyclodextrin, which can solve the problems of low accuracy, increased production of by-products, increased separation difficulty and the like , to achieve high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]Under stirring, dissolve 10 grams of β-cyclodextrin (8.8 mmol) in 20 mL of anhydrous dioxane solvent, and add 0.24 grams of sodium metal (10.6 mmol) in batches until the sodium metal is completely reacted, and add to the above reaction system 1.8 g of sultone (13.2 mmol) was slowly added dropwise, and the etherification reaction was carried out under reflux at 70° C. for 3 hours. After the reaction was complete, the amorphous solid was separated, washed with methanol three times, and dried. After drying, the solid was dissolved in 100 ml of deionized water, neutralized with 1N hydrochloric acid to pH 8, desalted and purified through a Sephadex column (G-25), the filtrate was concentrated, and freeze-dried to obtain white water-soluble sulfobutyl Ether-β-cyclodextrin 4.7 g (yield about 41%). The average molecular weight is 1307, elemental analysis: measured value C42.75%, H5.96%, S2.71%, Na1.93, C / S=15.77, calculated value (substitution degree is 1.1) C / S=15.7.
Embodiment 2
[0023] Under stirring, dissolve 10 grams of β-cyclodextrin (8.8 mmol) in 20 mL of anhydrous dioxane solvent, add 0.97 grams of sodium metal (42.3 mmol) in batches until the sodium metal is completely reacted, and then add to the above reaction system Slowly add 7.2 g of sultone (52.8 mmol) dropwise, and carry out etherification reaction at 90° C. for 3 hours under reflux reaction. After the reaction is complete, an amorphous solid is separated, washed with methanol three times, and dried. After drying, the solid was dissolved in 150 ml of deionized water, neutralized with 1N sulfuric acid to pH 8, desalted and purified through a Sephadex column (G-25), the filtrate was concentrated, and freeze-dried to obtain white water-soluble sulfobutyl Ether-β-cyclodextrin 8.3 g (yield about 52%). The average molecular weight is 1813, elemental analysis: measured value C39.35%, H5.65%, S7.49%, Na5.52, C / S=5.25, calculated value (substitution degree is 4.3) C / S=5.26.
Embodiment 3
[0025] Under stirring, dissolve 10 grams of β-cyclodextrin (8.8 mmol) in 20 mL of anhydrous dioxane solvent, and add 1.70 grams of sodium metal (73.9 mmol) in batches until the sodium metal is completely reacted, and then add to the above reaction system 12.57 g of sultone (92.4 mmol) was slowly added dropwise, and the reaction was carried out under reflux at 105° C. for etherification for 3 hours. After the reaction was complete, an amorphous solid was separated, washed with methanol three times, and dried. After drying, the solid was dissolved in 200 ml of deionized water, neutralized with 1N hydrochloric acid to pH 8, desalted and purified by ultrafiltration membrane permeation (500 MWCO fiber ester membrane), the filtrate was concentrated, and freeze-dried to obtain a slightly yellow water-soluble sulfonate Butyl ether-β-cyclodextrin 11.4 g (yield about 57%). The average molecular weight is 2271, elemental analysis: measured value C37.44%, H5.32%, S10.21%, Na7.26, C / S=3.67...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com