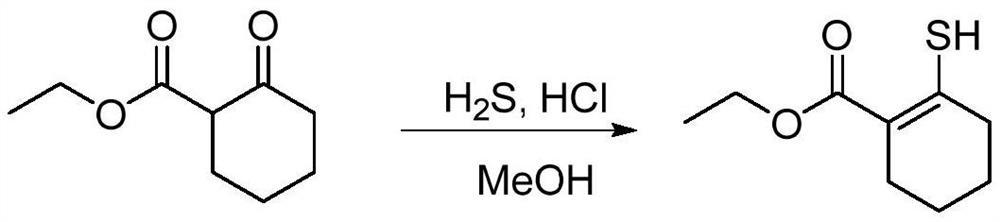
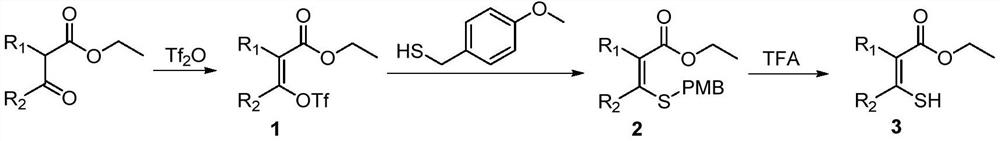
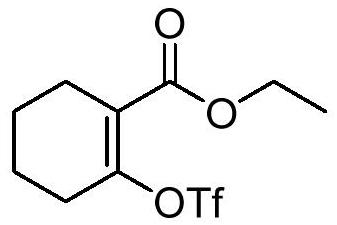
Preparation method of sulfydryl alkenyl ester compound
A technology of ester compounds and mercaptoalkenes, which is applied in the field of preparation of synthetic mercaptoalkene esters, can solve problems such as enlarged production risks and difficult safety control, so as to improve overall safety and environmental protection, solve industrialization problems, The effect of novel technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] (1) Synthesis of ethyl 2-(trifluoromethylsulfonyloxy)cyclohex-1-enecarboxylate (intermediate 1a, 122135-83-5)
[0024] In a 500mL three-neck flask, add 60% sodium hydrogen (3.5g, 1.5eq.), add 200mL dichloromethane, and replace with nitrogen three times. React at 0-5°C, add ethyl 2-cyclohexanone carboxylate (10.0 g, 1.0eq.) dropwise, keep stirring for 10 min, then continue to lower the temperature to -70°C, add Tf dropwise 2 O (24.3g, 1.5eq.) in the reaction solution (control the addition rate), keep stirring for 15 minutes, then naturally warm up to 25°C and stir overnight; then add saturated NaHCO 3 The pH of the solution was adjusted to 8-9, and the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain intermediate 1a (15.6 g, 90.0% yield).
[0025]
[0026] (2) Synthesis of ethyl 2-(4-methoxythiophenol)cyclohex-1-enecarboxylate (intermediate 2a)
[0027] In a 500 mL three-neck flask, add 60% sodium hydrogen (1.90 g, 1.2 eq....
Embodiment 2
[0032]
[0033] (1) Synthesis of ethyl 2-(trifluoromethylsulfonyloxy)cyclopent-1-enecarboxylate (intermediate 1b, 122539-74-6)
[0034] In a 500mL three-neck flask, add 60% sodium hydrogen (3.5g, 1.5eq.), add 200mL dichloromethane, and replace with nitrogen three times. React at 0-5°C, add ethyl 2-cyclopentanone carboxylate (9.2g, 1.0eq.) dropwise, keep stirring for 10min, then continue to drop the temperature to -70°C, and continue to add Tf 2 O (24.9g, 1.5eq.) in the reaction solution (control the addition rate), keep stirring for 15 minutes, then naturally warm up to 25°C and stir overnight; then add saturated NaHCO 3 The pH of the solution was adjusted to 8-9, and the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain intermediate 1b (14.9 g, 88.0% yield).
[0035]
[0036] (2) Synthesis of ethyl 2-(4-methoxythiophenol)cyclopent-1-enecarboxylate (intermediate 2b)
[0037] In a 500mL three-neck flask, add 60% sodium hydrogen (1.67g, 1.2...
Embodiment 3
[0042]
[0043] (1) Synthesis of ethyl 2-(trifluoromethylsulfonyloxy)cyclohep-1-enecarboxylate (intermediate 1c, 122539-74-6)
[0044] In a 500mL three-neck flask, add 60% sodium hydrogen (3.3g, 1.5eq.), add 200mL dichloromethane, and replace with nitrogen three times. React at 0-5°C, add ethyl 2-cycloheptanonecarboxylate (10.1g, 1.0eq.) dropwise, keep stirring for 10min, then continue to drop the temperature to -70°C, and continue to add Tf 2 O (23.2g, 1.5eq.) in the reaction solution (control the addition rate), keep stirring for 15 minutes, then naturally warm up to 25°C and stir overnight; then add saturated NaHCO 3 The pH of the solution was adjusted, the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain intermediate 1c (14.7 g, 85.0% yield).
[0045]
[0046](2) Synthesis of ethyl 2-(4-methoxythiophenol) cyclohept-1-enecarboxylate (intermediate 2c)
[0047] In a 500mL three-neck flask, add 60% sodium hydrogen (1.5g, 1.2eq.), add 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com