Chimeric antigen receptor and use thereof
A chimeric antigen receptor, dectin-1 technology, applied in the direction of antibody medical components, antibody mimics/scaffolds, carriers, etc., can solve the problem of lack of tumor-specific antigens, CAR-T cell exhaustion, damage to CAR-T cells Tumor effectiveness and other issues to achieve the effect of reducing exhaustion and increasing cell expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Materials and methods
[0052] Cell lines: All cell lines were derived from ATCC. Combine K562 cells (myeloid leukemia) and NALM6 cells (lymphocytic leukemia) in RPMI-1640 with heat-inactivated 10% fetal bovine serum (FBS) (PAN, Germany.Cat: ST30-3302), penicillin (100 U / mL) (Gibco, Thermo Fisher, Waltham, MA. Cat: SV30010) and streptomycin (100ug / mL) (Gibco, Thermo Fisher, Waltham, MA. Cat: SV30010) were cultured together. Human cancer cell lines SK-OV-3 (ovarian cystadenocarcinoma) and MDA-MB-468 (breast cancer) were treated with heat-inactivated 10% FBS, penicillin (100U / mL) and streptomycin (100ug / mL) cultured in DMEM. SK-OV-3 was also engineered to express luciferase.
[0053] 2. Construction of plasmid encoding CAR
[0054] Anti-CD19 or anti-HER2 CARs included single-chain variable fragments (scFv) specific for CD19 (clone FMC63) or HER2 (clone 4D5). The scFv is followed by the human CD8α hinge region, followed by the human CD8α transmembrane domain (TM),...
Embodiment 2
[0082] 1. Novel CAR structure with Dectin-1 costimulatory domain
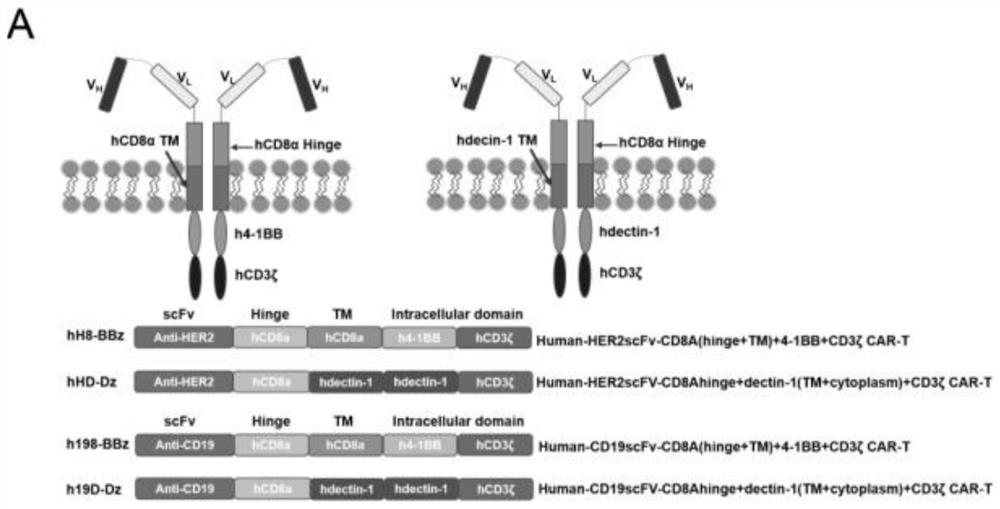
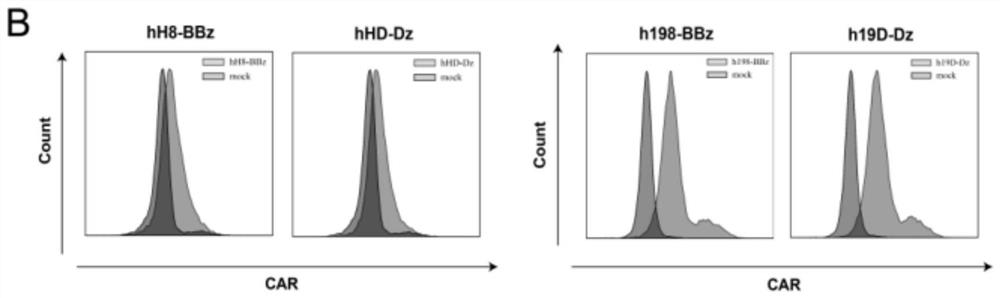
[0083] In the present invention, a novel second-generation CAR construct ( Figure 1A ). Two additional CAR constructs were generated using parts of the most popular second-generation CAR construct (human CD8αTM with 4-1BB and CD3δ ICDs). All constructed CARs were expressed on the surface of T cells ( Figure 1B ). Regarding CARs against HER2, the expression rate of hH8-BBz(4-1BB) CAR was 49.61%, and the expression rate of hHD-Dz(Dectin-1) CAR was 46.17%. Regarding the CARs against CD19, the expression rate of h198-BBz (4-1BB) CAR was 92.95%, and the expression rate of h19D-Dz (Dectin-1) CAR was 95.07%. Although the expression of anti-HER2 CARs was lower than that of anti-CD19 CARs, there was no significant difference in their expression levels among anti-HER2 or anti-CD19 CARs containing the same co-stimulatory signaling domain.
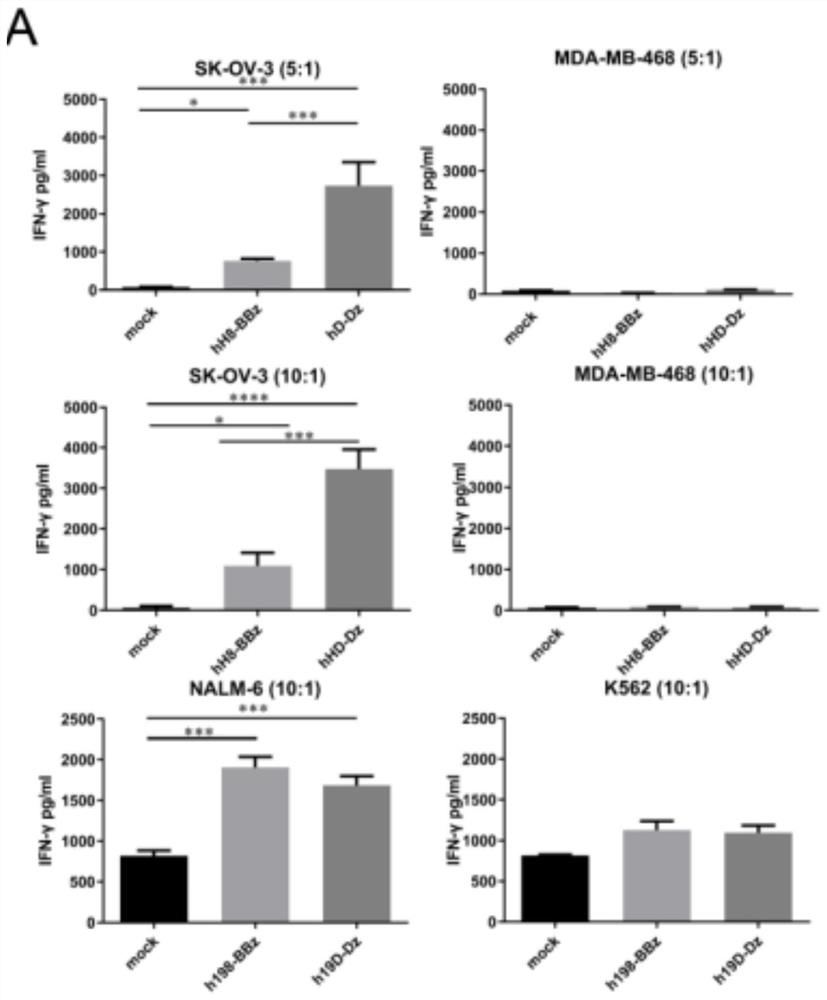
[0084] 2. Effect of Dectin-1 co-stimulation on the effector function of nov...
Embodiment 3
[0098] This embodiment also provides a co-stimulatory signaling domain of a chimeric antigen receptor, the co-stimulatory signaling domain comprising the intracellular region of RD-1.
[0099] Further, the co-stimulatory signal domain includes CD3δ.
[0100] Further, the intracellular region of RD-1 comprises the amino acid sequence of SEQ No.1 or an amino acid sequence not less than 90% identical to said SEQ No.1.
[0101] Further, the co-stimulatory signal domain also includes CD3, CD4, CD8, FcR, DAP10, DAP12, CD27, CD28, CD137, CD134, ICOS, OX40, CD30, CD40, PD-1, LFA-1, CD2, CD7 , LIGHI, NKG2C, B7-H3, ligands specifically binding to CD83, CDS, ICAM-1, GITR, BAFFR, HVEM (IGHTR), SLAMF7, NKp80 KLRE1, CD160, CD19, CD83, IL-2Rβ, IL-Rγ, IL-7Rα, ITGA4, VLA1, CD49α, ITGA4, IA4, CD49D, ITGA6, LA-6, CD49f, ITGAD, CD11d, ITGAE, CD103, ITGAL, CD11α, LFA-1, ITGAM, CDIIB, ITGAX, CD11c, ITGB1, CD29,ITGB2,CD18,LFA-1.ITGB7 TAFR2,TRANCE / RANKL,DNAM1(CD226),SLAMF4(CD244,2B4),CD84,CD96(Tact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com