Preparation method of aliphatic polycarbonate polyester copolymer without catalyst addition
A polycarbonate polyester and copolymer technology, which is applied in the field of material science, can solve the problems of inability to evaluate the safety of small molecular organic compounds, high catalyst toxicity, etc., and achieves great application and transformation potential. The preparation method is simple and efficient, and the preparation steps are simple. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The reaction formula prepared by aliphatic polycarbonate polyester copolymer is as follows:
[0050]
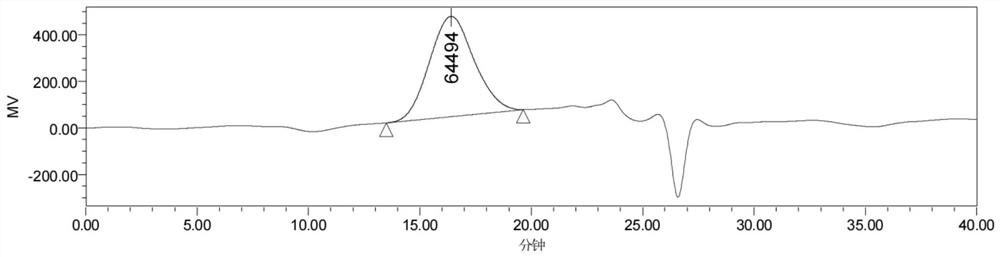
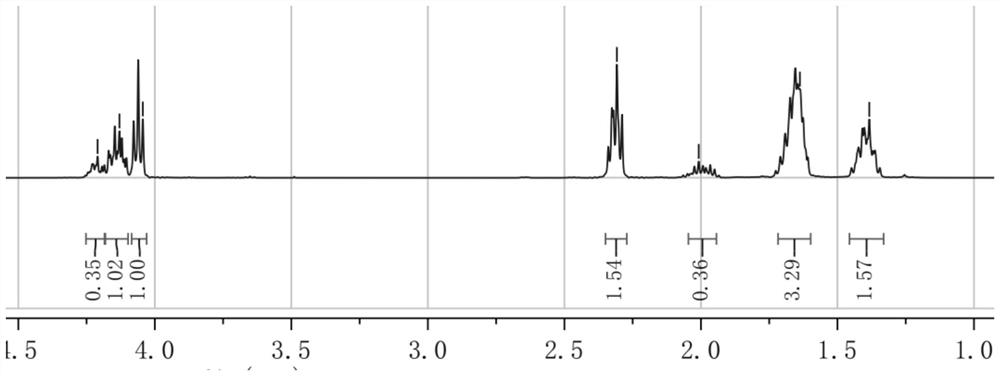
[0051] The preparation of aliphatic polycarbonate polyester copolymer specifically includes the steps of: weighing 0.15 gram of six-membered ring carbonate [specifically trimethylene carbonate (abbreviated as TMC)] and 0.15 cyclic lactone [specifically ε-hexyl Lactone (abbreviated as CL)] was added into a decompressible airtight container, dried in vacuum for 0.5 hour, and sealed under negative pressure. Under magnetic stirring, the reaction vessel was placed in an oil bath at 100° C. for 7 hours, and the polymerization was completed. The product was dissolved in 2mL of dichloromethane and dropped into 40mL of methanol to obtain a white precipitate. The methanol was poured while washing the product 2 times with clean methanol. After drying, an aliphatic polycarbonate polyester copolymer is obtained, whose structural formula is Weigh, calculate productive rate, p...
Embodiment 2
[0054] The reaction formula that aliphatic polycarbonate polyester copolymer prepares is with embodiment 1, and the preparation of aliphatic polycarbonate polyester copolymer specifically comprises the steps:
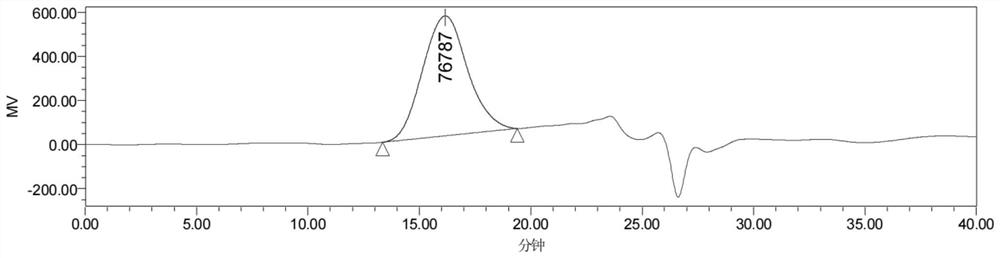
[0055] Weigh 0.15 g of six-membered ring carbonate [specifically trimethylene carbonate (abbreviated as TMC)] and 0.15 cyclic lactone [specifically ε-caprolactone (abbreviated as CL)] and add it to a decompressible airtight container, Vacuum dry for 0.5 hours, and seal under negative pressure. Under magnetic stirring, the reaction vessel was placed in an oil bath at 120° C. for 7 hours, and the polymerization was completed. The product was dissolved in 2mL of dichloromethane and dropped into 40mL of methanol to obtain a white precipitate. The methanol was poured while washing the product 2 times with clean methanol. After drying, an aliphatic polycarbonate is obtained, whose structural formula is Weigh, calculate productive rate, productive rate is 38%. GPC (THF as...
Embodiment 3
[0057] The reaction formula that aliphatic polycarbonate polyester copolymer prepares is with embodiment 1, and the preparation of aliphatic polycarbonate polyester copolymer specifically comprises the steps:
[0058] Weigh 0.15 g of six-membered ring carbonate [specifically trimethylene carbonate (abbreviated as TMC)] and 0.15 cyclic lactone [specifically ε-caprolactone (abbreviated as CL)] and add it to a decompressible airtight container, Vacuum dry for 0.5 hours, and seal under negative pressure. Under magnetic stirring, the reaction vessel was placed in an oil bath at 140° C. for 7 hours, and the polymerization was completed. The product was dissolved in 2mL of dichloromethane and dropped into 40mL of methanol to obtain a white precipitate. The methanol was poured while washing the product 2 times with clean methanol. After drying, an aliphatic polycarbonate is obtained, whose structural formula is Weigh, calculate productive rate, productive rate is 41%. GPC (THF as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com