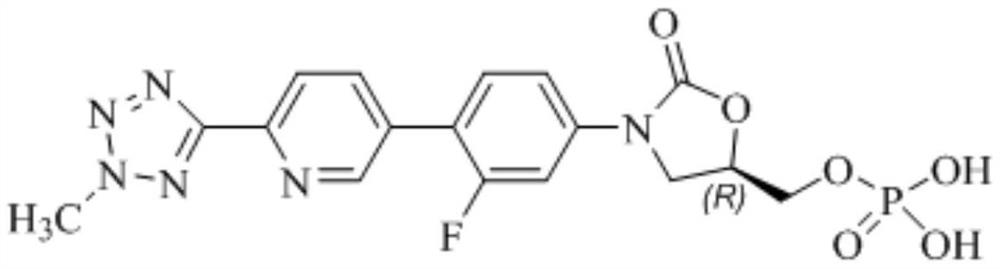
Tedizolid phosphate oral solid preparation
A technology of tedizolid phosphate and solid preparations, which is applied in the direction of pill delivery, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. It can solve problems such as poor dissolution effect and poor fluidity of tedizolid phosphate , to achieve the effect of ensuring product quality and process smoothness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
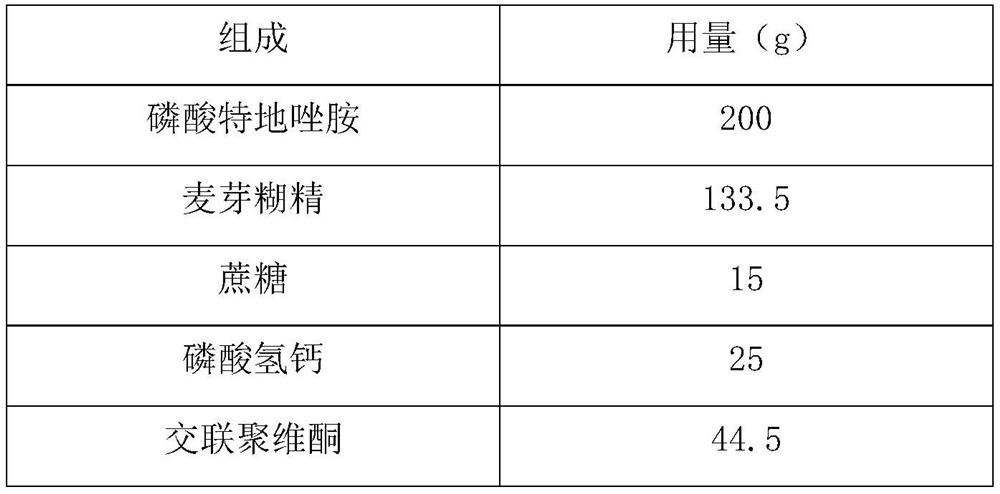
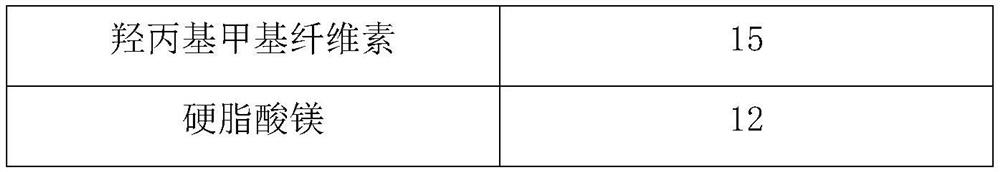
[0030] composition Dosage (g) Tedizolid Phosphate 200 Maltodextrin 120 Calcium hydrogen phosphate 10 Croscarmellose Sodium 20
[0031] Tedizolid phosphate d0.9 = 18 μm, calcium hydrogen phosphate d0.9 = 15 μm
[0032] Preparation:
[0033] (1) uniformly mixing tedizolid phosphate, maltodextrin, calcium hydrogen phosphate, and croscarmellose sodium to prepare a mixture;
[0034] (2) Add 50% ethanol aqueous solution to the mixture in step (1), granulate, granulate, and obtain drug granules;
[0035] (3) Compressing the drug granules into tablets.
Embodiment 2
[0037] composition Dosage (g) Tedizolid Phosphate 153 Maltodextrin 120 Calcium hydrogen phosphate 15 Low-substituted hydroxypropyl cellulose 8 Crospovidone 4
[0038] Tedizolid phosphate d0.9 = 25 μm, calcium hydrogen phosphate d0.9 = 20 μm
[0039] Preparation:
[0040] (1) uniformly mixing tedizolid phosphate, maltodextrin, calcium hydrogen phosphate, low-substituted hydroxypropyl cellulose, and crospovidone to prepare a mixture;
[0041] (2) Add 75% ethanol aqueous solution to the mixture in step (1), granulate and granulate to obtain drug granules;
[0042] (3) Compressing the drug granules into tablets.
Embodiment 3
[0044] composition Dosage (g) Tedizolid Phosphate 100 Maltodextrin 75 pregelatinized starch 30 Calcium hydrogen phosphate 15 Croscarmellose Sodium 20 povidone 10
[0045] Tedizolid phosphate d0.9 = 16 μm, calcium hydrogen phosphate d0.9 = 8 μm
[0046] Preparation:
[0047] (1) uniformly mixing tedizolid phosphate, maltodextrin, pregelatinized starch, calcium hydrogen phosphate, croscarmellose sodium and povidone to prepare a mixture;
[0048] (2) Add 60% ethanol aqueous solution to the mixture in step (1), granulate, granulate, and obtain drug granules;
[0049] (3) Compressing the drug granules into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com