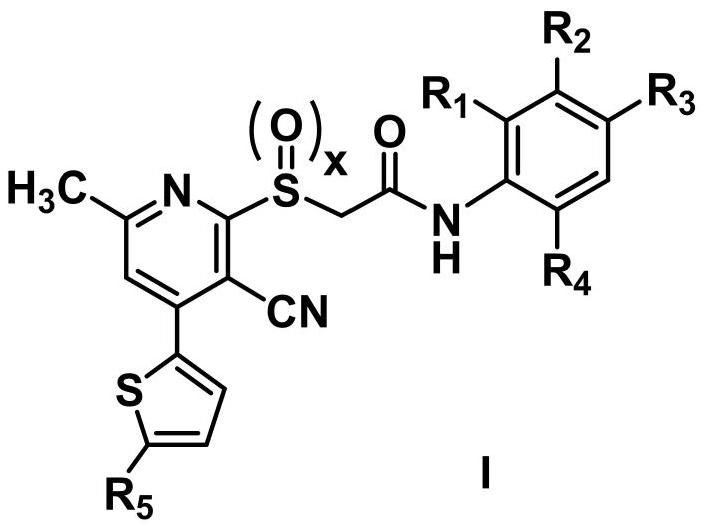
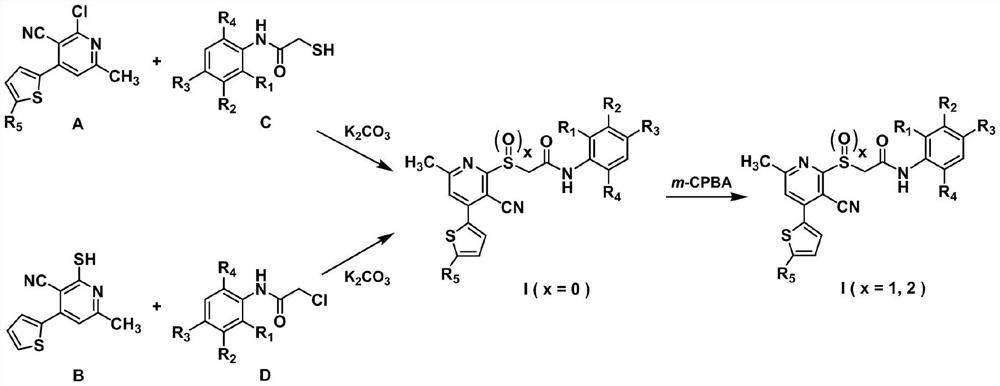
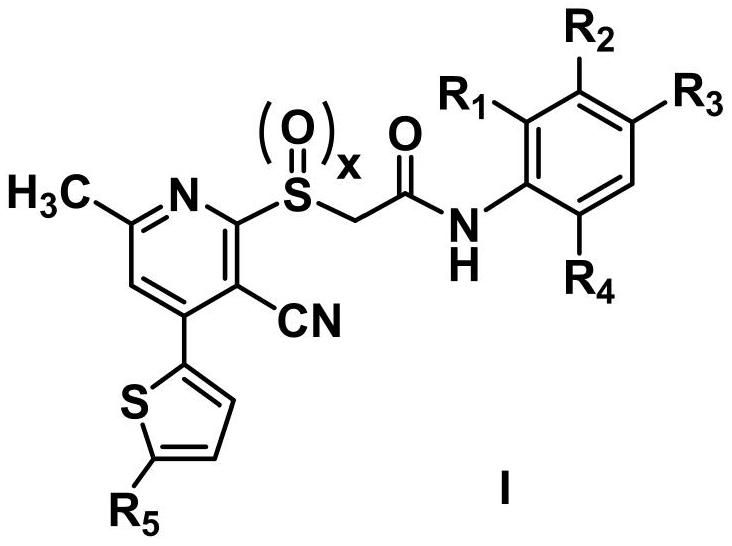
Acetylamine derivative containing thienyl pyridine and sulfur group and preparation method and application thereof
A technology of thienylpyridine and acetoarylamide is applied in the field of acetoarylamide derivatives and their preparation, which can solve the problems of the preparation of pyrazolecarboxamide derivatives and the undisclosed insecticidal activity, and achieves high insecticidal activity, good insecticidal activity, and the like. Control the effect of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Compound I-3 (2-((3-cyano-6-methyl-4-(thiophen-2-yl)pyridin-2-yl)thio)-N-(4-fluorophenyl)acetamide) method of preparation.
[0038] The reaction scheme is shown in the following formula:
[0039]
[0040] The preparation method is as follows:
[0041] Step 1: Preparation of 2-chloro-6-methyl-4-(thiophen-2-yl)nicotinonitrile (compound of formula A1)
[0042]
[0043] Prepared by referring to the method in the literature (J.Chem., 2013, 2013: 1-7; ACS Omega, 2019, 4: 8406-8412).
[0044] Add ethanol (250mL), 2-thiophenecarbaldehyde (11.22g), ethyl cyanoacetate (11.31g) and acetone (5.81g) into a 500mL two-necked flask in turn, and add ammonium acetate (23.12g) while stirring at room temperature. The mixture was heated to reflux for 12 hours, then cooled to room temperature, a solid precipitated, filtered, washed with ethanol, and dried to give a yellow solid 6-methyl-2-oxo-4-(thiophen-2-yl)-1,2- Dihydropyridine-3-carbonitrile was directly put into the next react...
Embodiment 2
[0054] Compound I-11 (2-((3-cyano-6-methyl-4-(thiophen-2-yl)pyridin-2-yl)thio)-N-(2,4,6-trichlorobenzene base) the preparation method of acetamide).
[0055] The reaction scheme is shown in the following formula:
[0056]
[0057] The preparation method is as follows:
[0058] Step 1: Preparation of 2-mercapto-6-methyl-4-(thiophen-2-yl)nicotinonitrile (compound of formula B1)
[0059]
[0060] Prepared according to the method in the literature (J.Chem., 2013, 2013: 1-7).
[0061] The compound of formula A1 (2-chloro-6-methyl-4-(thiophen-2-yl)nicotinonitrile) (7.04g), ethanol (100mL), and thiourea (4.57g) were sequentially added into a 250mL two-necked bottle, Heated to reflux and reacted for 8 hours. The system was cooled to room temperature, a solid was precipitated, filtered, washed with ethanol, and dried to obtain 2-mercapto-6-methyl-4-(thiophen-2-yl)nicotinonitrile (compound of formula B1) as a yellow solid.
[0062] Step 2: Preparation of 2-chloro-N-(2,4,6-tri...
Embodiment 3
[0070] Compound I-12 (2-((3-cyano-6-methyl-4-(5-methylthiophen-2-yl)pyridin-2-yl)thio)-N-(3-fluorophenyl ) the preparation method of acetamide).
[0071] The reaction scheme is shown in the following formula:
[0072]
[0073] The preparation method is as follows:
[0074] Step 1: Preparation of 2-chloro-6-methyl-4-(5-methylthiophen-2-yl)nicotinonitrile (compound of formula A2)
[0075]
[0076] Prepared by referring to the method in the literature (J.Chem., 2013, 2013: 1-7; ACS Omega, 2019, 4: 8406-8412).
[0077] Add ethanol (250mL), 5-methylthiophene-2-carbaldehyde (12.62g), ethyl cyanoacetate (11.31g) and acetone (5.81g) into a 500mL two-necked flask in sequence, and add ammonium acetate ( 23.12g). The mixture was heated to reflux for 12 hours, then cooled to room temperature, a solid was precipitated, filtered, washed with ethanol, and dried to obtain a yellow solid, which was directly put into the next reaction.
[0078] 6-methyl-4-(5-methylthiophen-2-yl)-2-ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com