Orally disintegrating tablet containing trihexyphenidyl hydrochloride and folic acid and its preparation method
A technology of trihexyphenidyl hydrochloride and trihexyphenidyl acid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The embodiment of the present invention provides a preparation method of an orally disintegrating tablet containing trihexyphenidyl hydrochloride and folic acid, comprising the following steps:
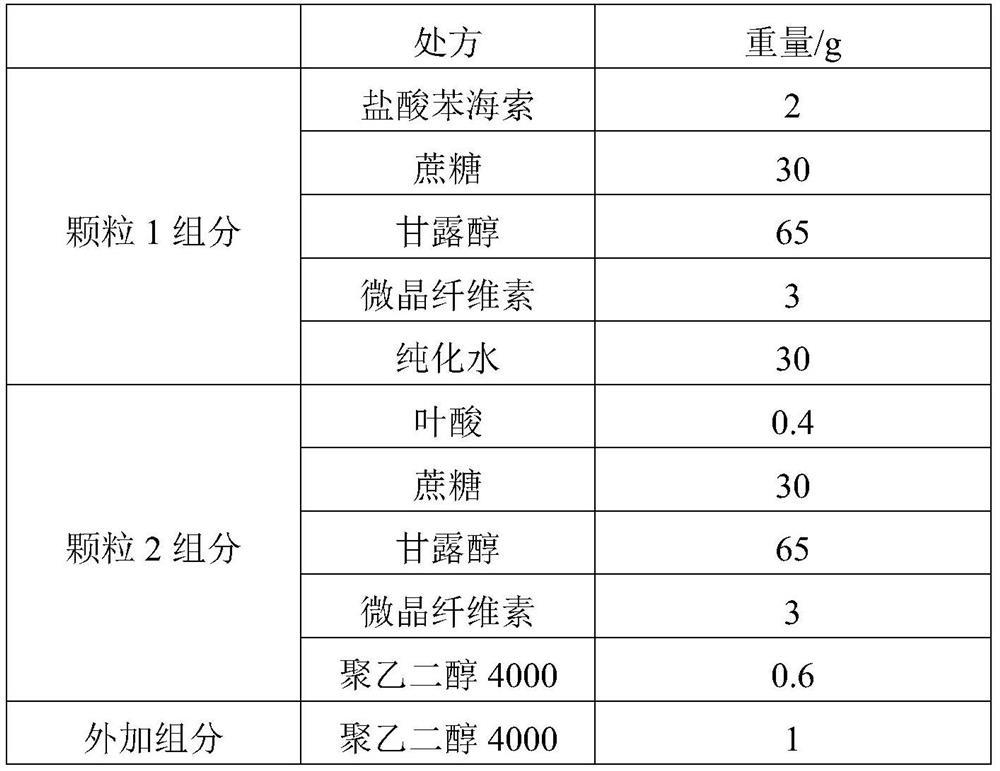
[0035] (a) Preparation of trihexyphenidyl hydrochloride granules: granulate trihexyphenidyl hydrochloride, microcrystalline cellulose, the first solid auxiliary material and water, the first solid auxiliary material is a mixture of sugar and alcohol, and the trihexyphenidyl hydrochloride The mass fraction of the solid component is 2% to 5%, and the mass fraction of the microcrystalline cellulose in the solid component is 2% to 4%;
[0036] (b) Preparation of folic acid granules: Folic acid, microcrystalline cellulose, second solid auxiliary material and polyethylene glycol are granulated, the second solid auxiliary material is a mixture of sugar and alcohol, and the folic acid accounts for the folic acid granule component The mass fraction is 0.4%-5%, the microcrystalline cellu...
Embodiment 1
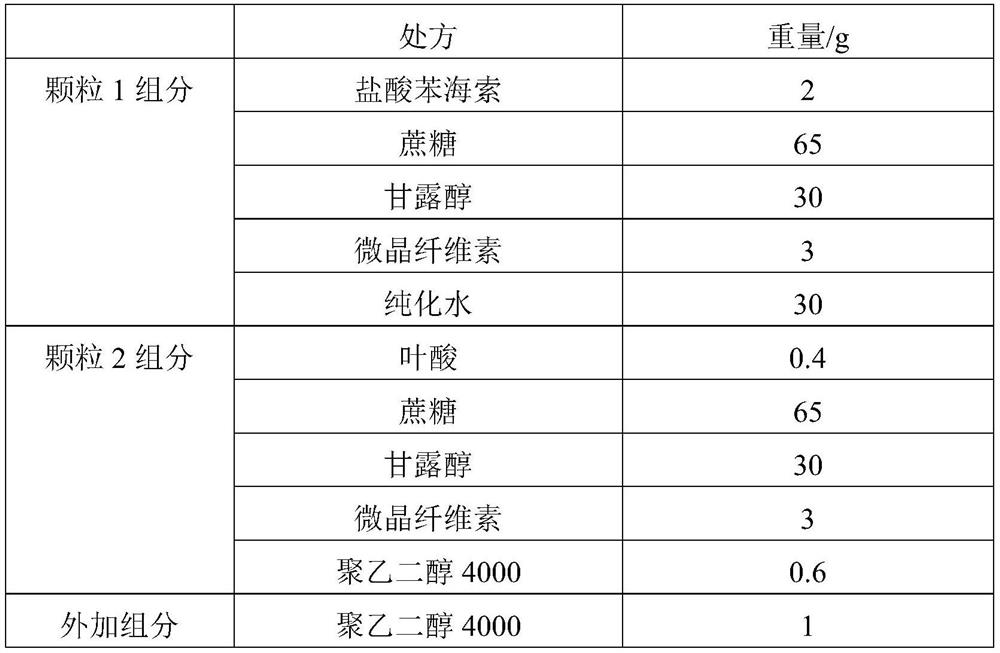
[0064] The group assignments are shown in Table 1.
[0065] Table 1
[0066]
[0067] Process:
[0068] (1) Pulverization: Mechanical pulverization of trihexyphenidyl hydrochloride to measure the particle size; airflow pulverization of folic acid to measure the particle size; mechanical pulverization of sucrose, passing through an 80-mesh sieve, and set aside.
[0069] (2) Preparation of trihexyphenidyl hydrochloride granules: add trihexyphenidyl hydrochloride, sucrose, mannitol and microcrystalline cellulose to a wet granulator and mix, add a wetting agent and purified water to make a soft material, granulate with 20 mesh, 50-60 °C Fluidized bed drying, 20 mesh granulation;
[0070] (3) Preparation of folic acid granules: Folic acid, sucrose, mannitol and microcrystalline cellulose are first mixed in a wet granulator, then mixed with polyethylene glycol 4000 in a three-dimensional mixer, then dry granulated, granulated at 20 mesh;
[0071] (4) Total mixing: add trihexyp...
Embodiment 2
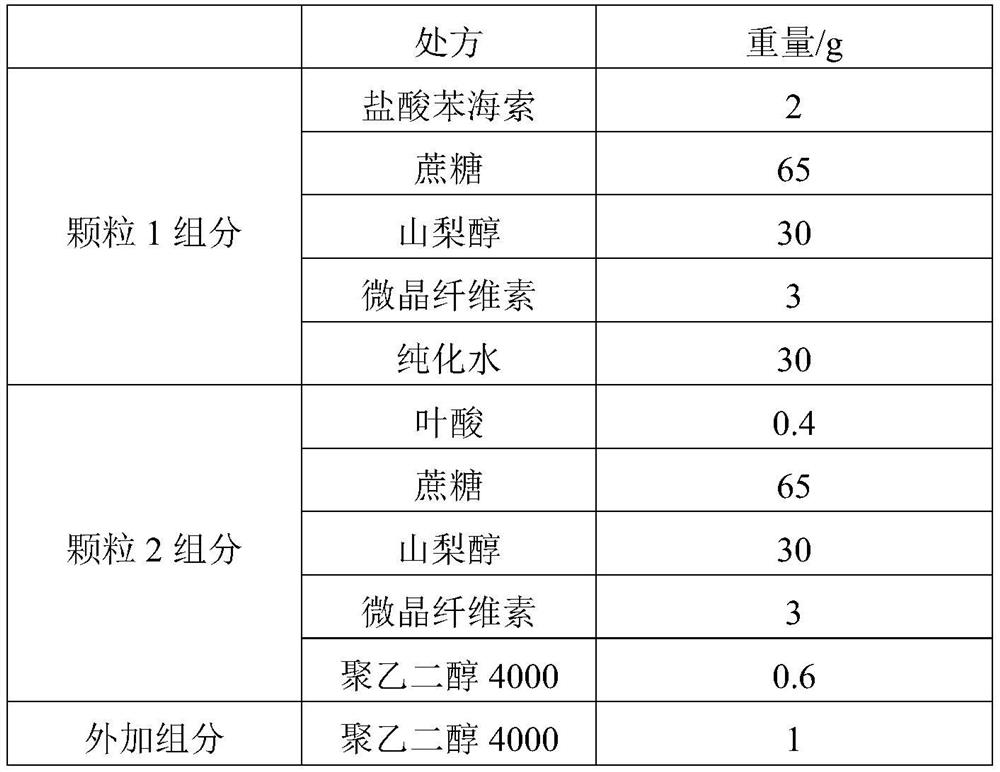
[0074] The group assignments are shown in Table 2.
[0075] Table 2
[0076]
[0077] (1) Pulverization: Mechanical pulverization of trihexyphenidyl hydrochloride to measure the particle size; airflow pulverization of folic acid to measure the particle size; mechanical pulverization of sucrose, passing through an 80-mesh sieve, and set aside.
[0078] (2) Preparation of trihexyphenidyl hydrochloride granules: Add trihexyphenidyl hydrochloride, sucrose, sorbitol and microcrystalline cellulose to a wet granulator and mix, add a wetting agent and purified water to make soft materials, granulate with 20 mesh, 50-60°C Fluidized bed drying, 20 mesh granulation;
[0079] (3) Preparation of folic acid granules: Folic acid, sucrose, sorbitol and microcrystalline cellulose are first mixed in a wet granulator, then mixed with polyethylene glycol 4000 in a three-dimensional mixer, then dry granulated, granulated at 20 mesh;
[0080] (4) Total mixing: add trihexyphenidyl hydrochloride...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com