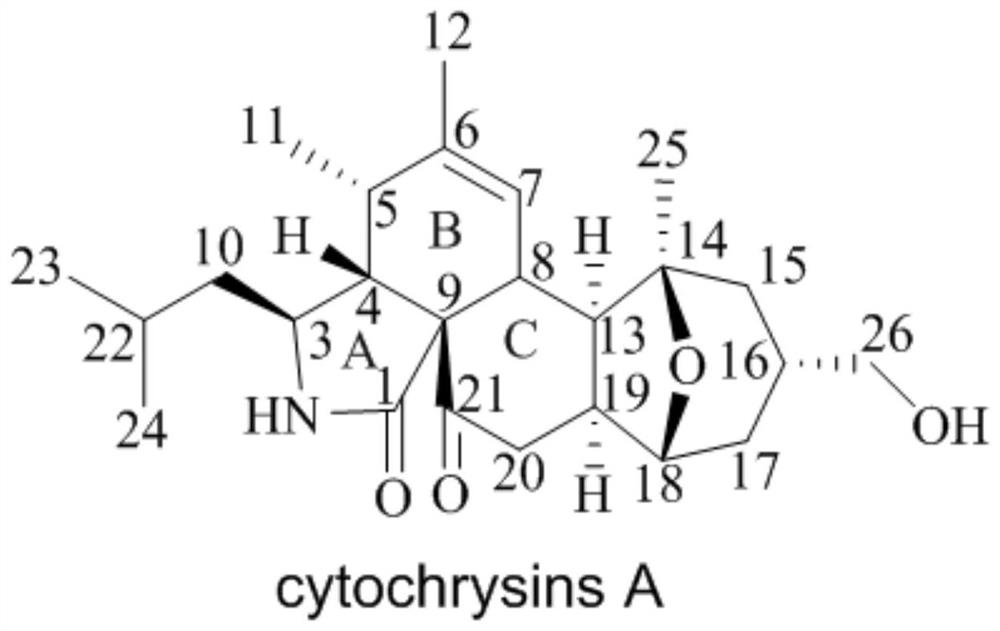
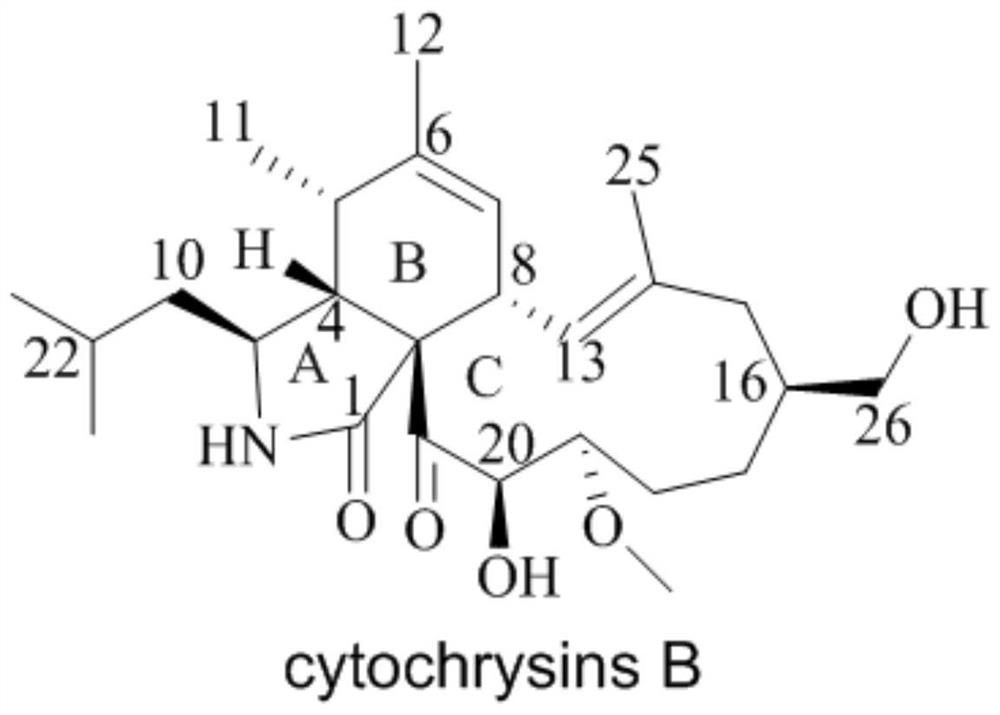
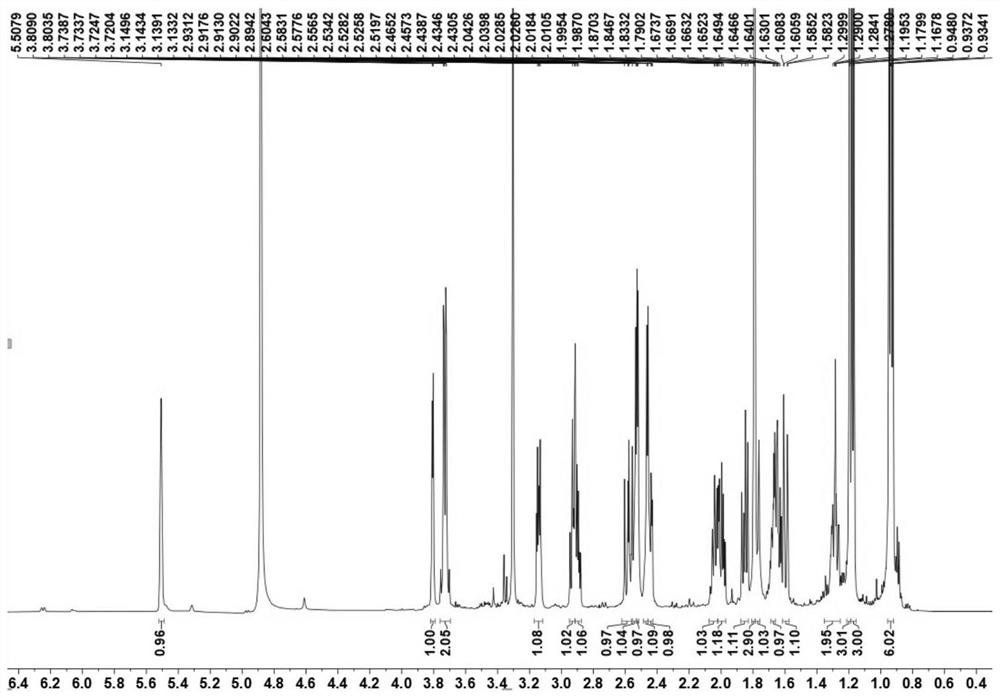
Novel cytochalasin compound with function of antagonizing clinical drug-resistant bacteria and preparation method of novel cytochalasin compound
A technology of cytochalasin and compounds, which is applied in the field of biomedicine and can solve the problems reported by scholars on the antagonism of cytochalasin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A method for preparing a novel cytochalasin compound with the function of antagonizing clinical drug-resistant bacteria, comprising the following steps:
[0055] Step (1), the fermentation culture of Ascocystis aureus;
[0056] In step (2), methanol is used to extract the secondary metabolites obtained by the fermentation and cultivation of Ascocystis aureus, concentrated under reduced pressure to evaporate the solvent, washed with water, then extracted with ethyl acetate, and finally concentrated under reduced pressure to obtain a crude extract;
[0057] In step (3), the crude extract is wet-loaded to a silica gel column, and through silica gel column chromatography, gradient elution is performed with a mixed organic solvent of dichloromethane and methanol at a volume ratio of 100:1-1:1, and each sample is collected. The gradient eluate of the gradient was concentrated and monitored by TLC, and the same fractions were combined to obtain six groups of segments Fr.A-Fr.F...
Embodiment 2
[0066] A method for preparing a novel cytochalasin compound with the function of antagonizing clinical drug-resistant bacteria, comprising the following steps:
[0067] Step (1), the fermentation culture of Ascocystis aureus;
[0068] In step (2), methanol is used to extract the secondary metabolites obtained by the fermentation and cultivation of Ascocystis aureus, concentrated under reduced pressure to evaporate the solvent, washed with water, then extracted with ethyl acetate, and finally concentrated under reduced pressure to obtain a crude extract;
[0069] In step (3), the crude extract is wet-loaded to a silica gel column, and through silica gel column chromatography, gradient elution is performed with a mixed organic solvent of dichloromethane and methanol at a volume ratio of 100:1-1:1, and each sample is collected. The gradient eluate of the gradient was concentrated and monitored by TLC, and the same fractions were combined to obtain six groups of segments Fr.A-Fr.F...
Embodiment 3
[0083] A method for preparing a novel cytochalasin compound with the function of antagonizing clinical drug-resistant bacteria, comprising the following steps:
[0084] Step (1), the fermentation culture of Ascocystis aureus;
[0085] In step (2), methanol is used to extract the secondary metabolites obtained by the fermentation and cultivation of Ascocystis aureus, concentrated under reduced pressure to evaporate the solvent, washed with water, then extracted with ethyl acetate, and finally concentrated under reduced pressure to obtain a crude extract;
[0086] In step (3), the crude extract is wet-loaded to a silica gel column, and through silica gel column chromatography, gradient elution is performed with a mixed organic solvent of dichloromethane and methanol at a volume ratio of 100:1-1:1, and each sample is collected. The gradient eluate of the gradient was concentrated and monitored by TLC, and the same fractions were combined to obtain six groups of segments Fr.A-Fr.F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com