Immunomodulating compositions from bile
An immunomodulatory agent and composition technology, which is applied in the directions of drug combination, hydrocarbon compound active ingredient, hydroxyl compound active ingredient, etc., can solve the problem of bile not considering tumors or infectious diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] The preparation of embodiment 1 composition of the present invention
[0224] Bile is collected from healthy cattle at least one and a half years of age slaughtered for food by a licensed and inspected slaughterhouse. Gallbladders were collected from self-examined slaughtered animals, the gallbladders were separated from the livers and veterinary examination confirmed that the gallbladders were free of parasites and showed no signs of infection, therefore the gallbladders were suitable for use as a source of bile.
[0225] The gallbladder that passed the test was wiped clean with 70% ethanol, and the bile was drawn out with a syringe. The withdrawn bile in the syringe was visually inspected by a veterinarian to ensure that it contained no blood or pus and was otherwise satisfactory. Transfer a satisfactory amount of bile into a graduated amber bottle of ethanol. Bile is a green liquid that is mostly free of blood and pus. Add bile up to twice th...
Embodiment 2
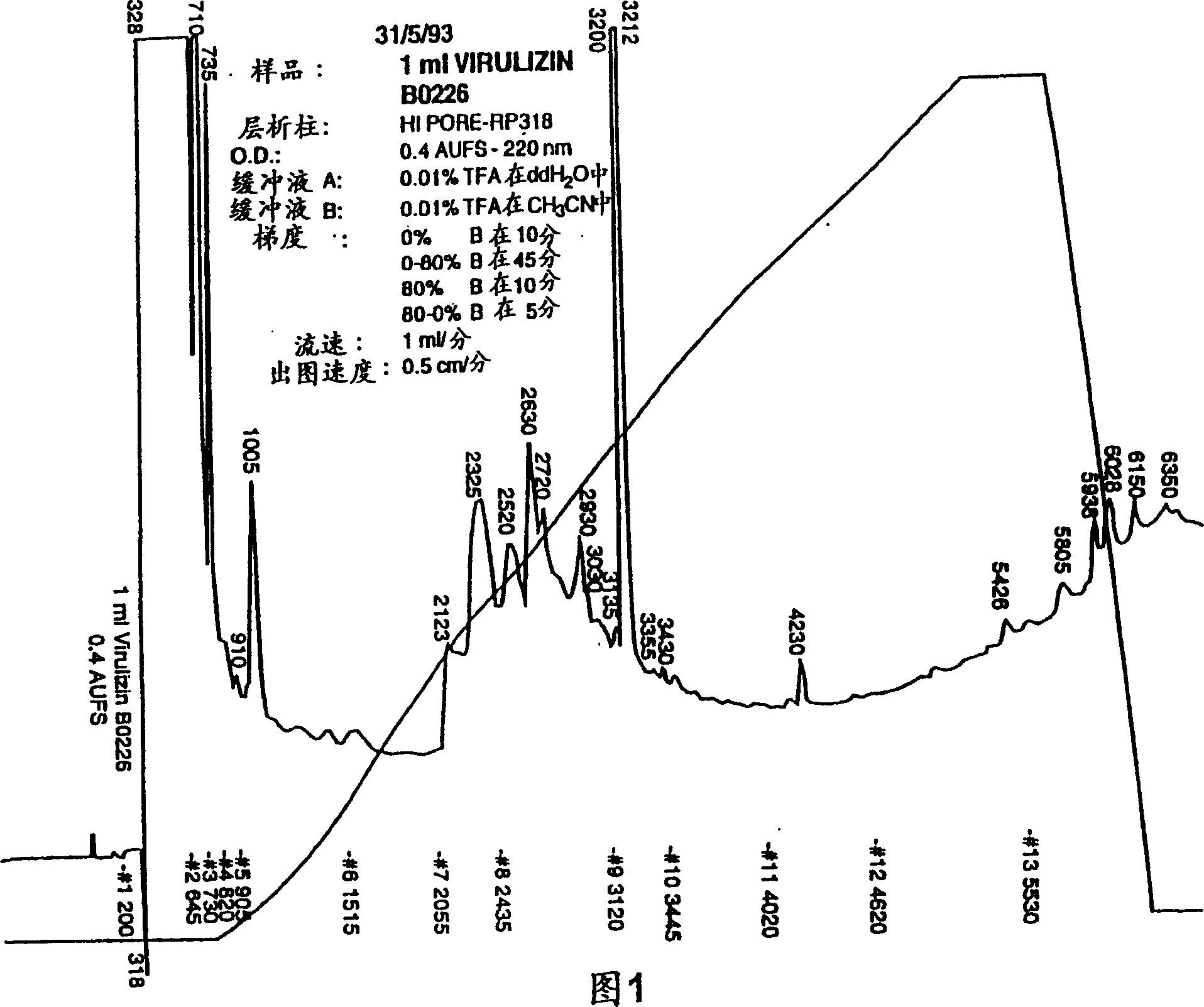
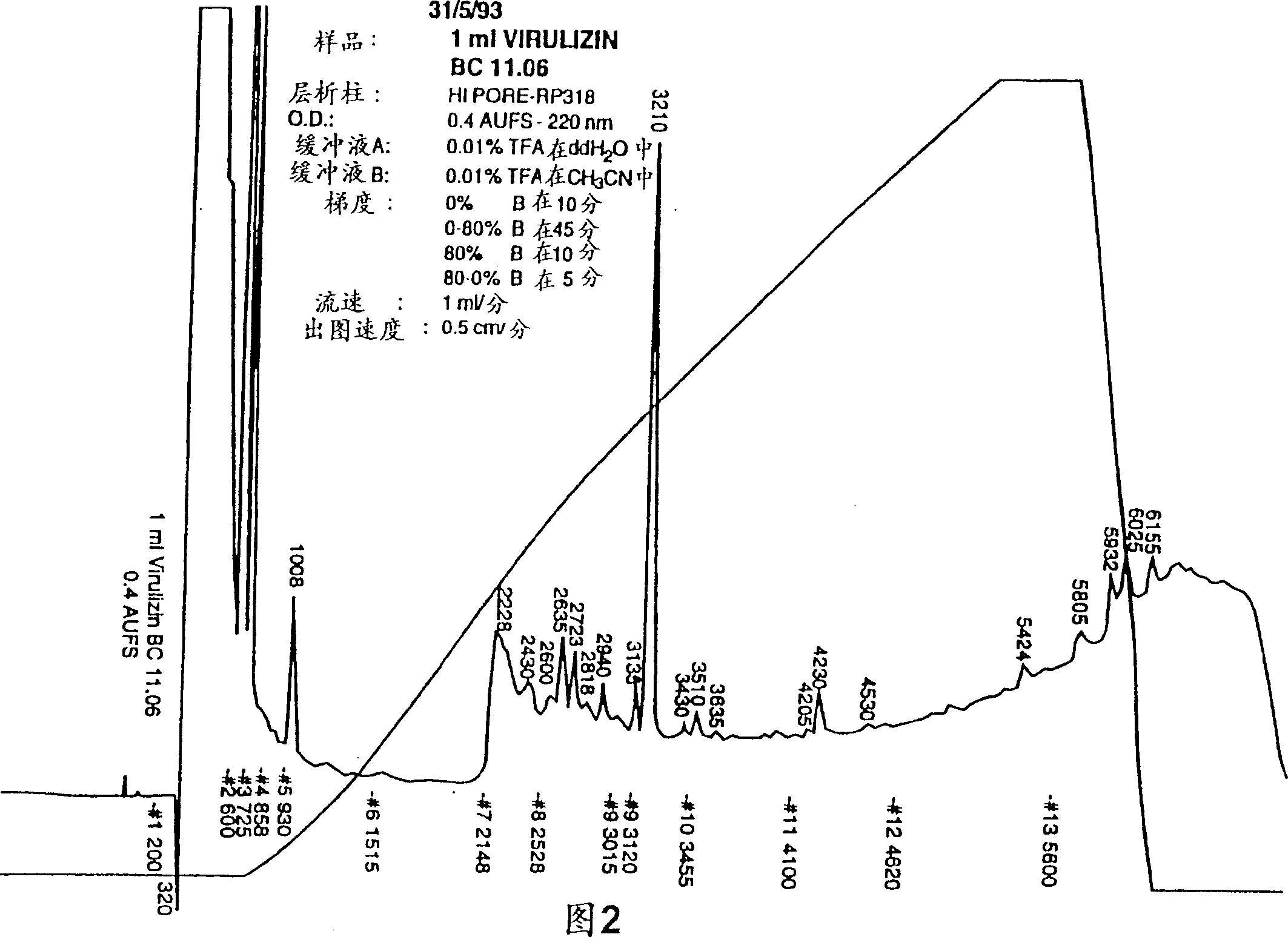
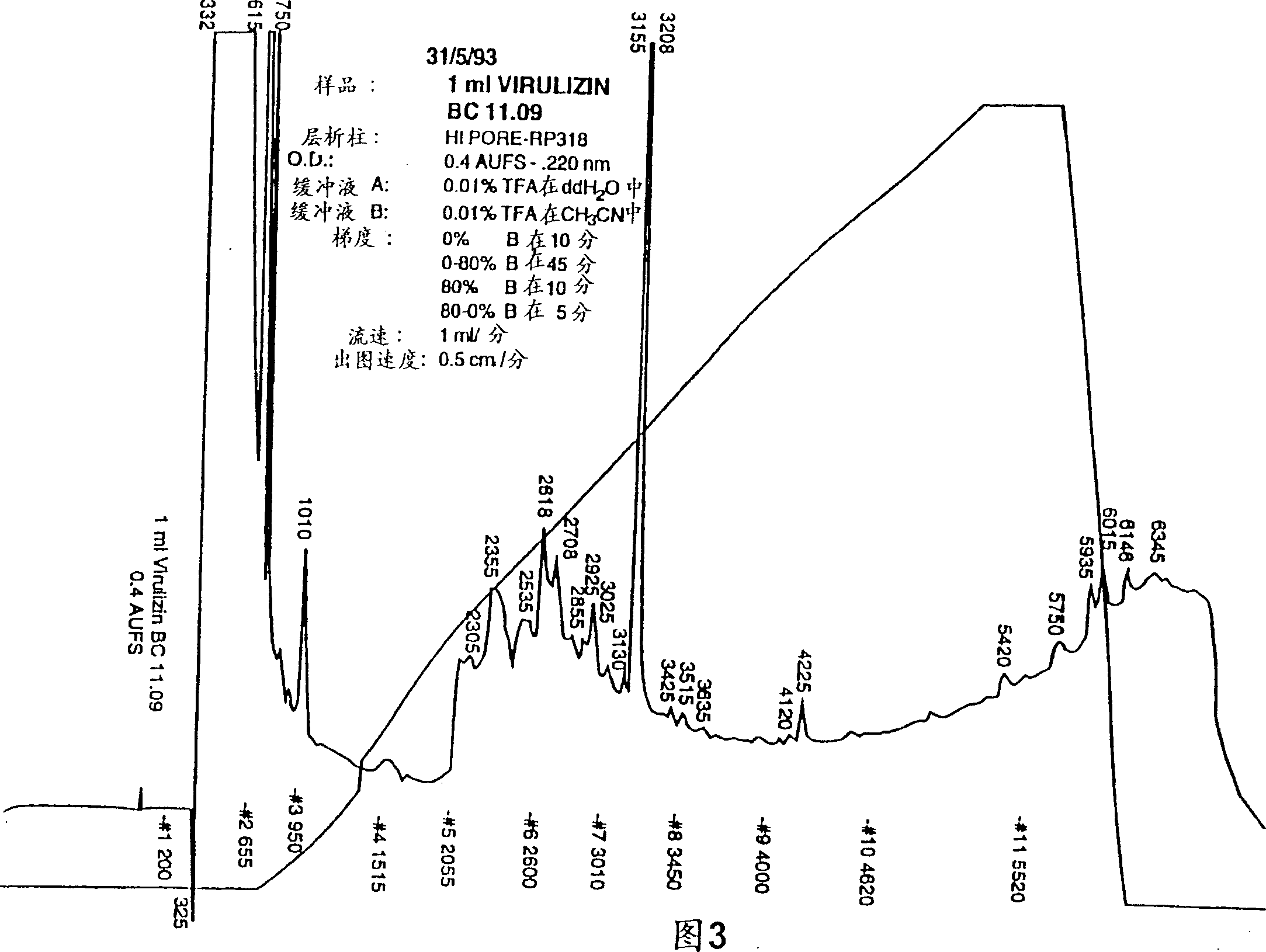
[0236] Example 2 Physicochemical and Biochemical Properties of Compositions of the Invention
[0237] A number of physicochemical properties (conductivity, osmotic pressure and total solids) of the preparations are shown in Table 2. More particularly, the results of the examination of three production batches of the composition prepared according to the method of Example 1 were made. The results shown in Table 2 demonstrate the sterility, potency and reproducibility of the products produced. Please note that ethanol and ether are only measured in the test. Example 4 provides a summary of methods for assaying biological activity.
[0238] Table 2: Product Specification Inspection Specification Method Biological activity 18+ / -5 units / ml Biological activity identity / purity Consistent with the reference HPLC Safety Passed inspection General safety inspection
[0239] (mice and guinea pigs)
...
Embodiment 3
[0259] The biological activity of embodiment 3 composition components
[0260] The biological activity of the composition fractions was studied. The biological activity of the composition is believed to be due to small molecular weight components (molecular weight less than 3000 Da). This conclusion was confirmed experimentally by testing the bioactivity of the four composition fractions and the unfractionated composition. The first fraction contains proteins and peptides with a molecular weight less than 3000 Da, while the remaining three fractions contain proteins and peptides with an additional larger molecular weight. All fractions contained additional larger molecular weight proteins and polypeptides. All fractions and unfractionated compositions showed the same biological activity. Since all fractions are as potent as the unfractionated product, and since a common feature of all fractions is the presence of molecules less than 3000 Da in the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com