Compound application
A technology of drugs and chemical components, applied in the field of biomedicine, can solve problems such as low safety and unsatisfactory drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 antitumor experiment
[0059] Experimental animals: 18-22g clean grade male mice.
[0060] experimental method:
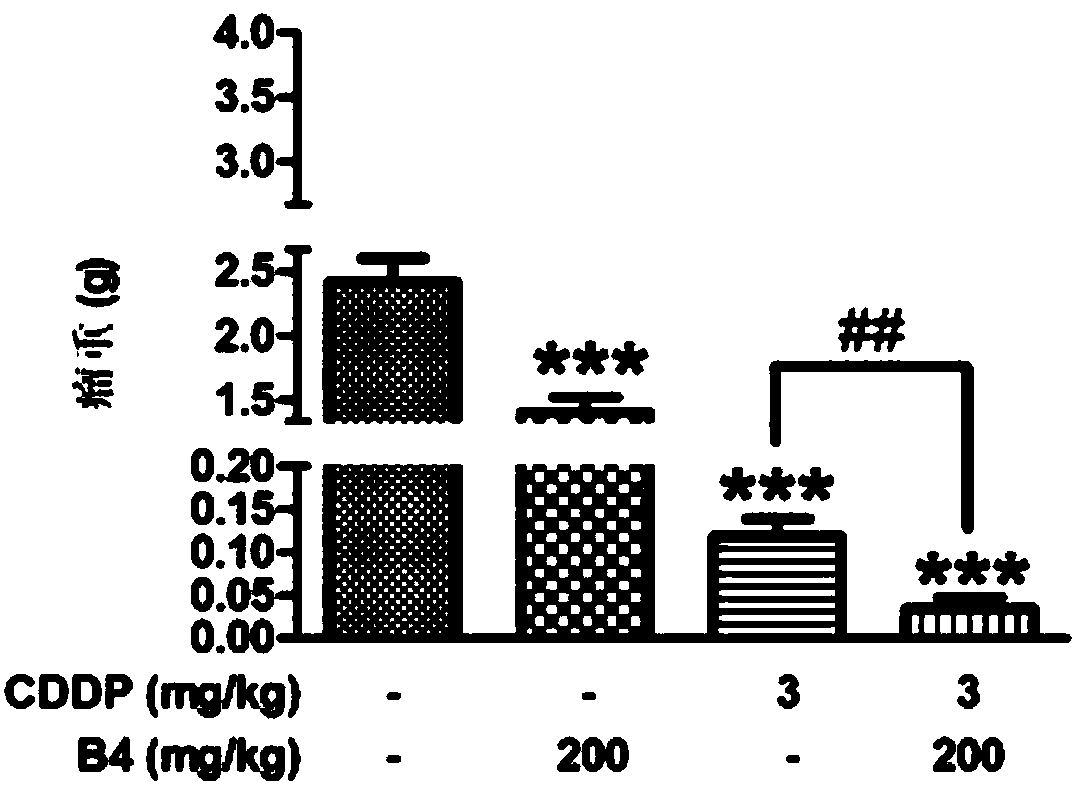
[0061] Set the cell density to 10 7 Each / mL S180 cell suspension was placed on ice. When inoculating, clamp the right forelimb of the mouse with tweezers and straighten it. Insert the needle from the right side of the mouse body at a distance of 1.5 cm from the armpit, and pass through the head without piercing the skin or muscle layer. When the needle reaches the armpit, inject , the volume of the injected cell suspension is 2mL / mouse, withdraw the needle quickly after the injection and release the mouse. After the inoculation, the mice were randomly divided into 4 groups, namely the model group, Pulsatilla saponin B4 (200mg / kg) group, cisplatin group (CDDP, 3mg / kg), cisplatin (3mg / kg) + Pulsatilla saponin B4 (200mg / kg) group, 13 rats in each group. 24 hours after inoculation, Pulsatilla saponin B4 group and cisplatin group were injected i...
Embodiment 2
[0063] Effect of Example 2 on in vitro hemolysis
[0064] Preparation of 2% erythrocyte suspension Take 10ml of fresh rabbit blood, put it into a Erlenmeyer flask filled with glass beads and shake it for 10 minutes, or stir the blood with a glass rod to remove fibrous protein and make it into defibrinated blood. Add 100ml of normal saline, shake well, centrifuge at 1500r / min for 15 minutes, remove the supernatant, and wash the precipitated erythrocytes with normal saline for 2-3 times according to the above method until the supernatant does not appear red. The obtained erythrocytes were mixed with physiological saline to form a 2% suspension (2 ml of erythrocytes, added with physiological saline to make 100 ml) for the test.
[0065] Take 6 clean glass test tubes of 10ml and number them. Tubes 2 to 4 are the test samples, tube 1 is the negative control tube, and tube 6 is the positive control tube (complete hemolysis control). Sequentially add Pulsatilla saponin B4 at a final...
Embodiment 3
[0072] Embodiment 3 safety evaluation
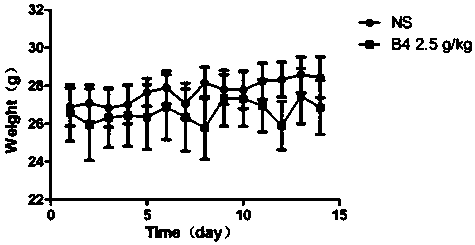
[0073] The safety of Pulsatilla saponin B4 was evaluated by acute toxicity test. The 18-25g ICR mice were randomly divided into B4 administration group and normal saline group, ten in each group. The mice in the Pulsatilla saponin B4 administration group were intraperitoneally injected with 2.5g / kg Pulsatilla saponin B4 every day, and the normal saline group was injected with the same amount of normal saline for 14 consecutive days. The mice were weighed every day to observe the survival status of the mice. The mice were sacrificed on the 14th day of administration, and the serum was extracted to detect the relevant indexes of liver and kidney function. The experimental results showed that, compared with the blank control group, there was no significant difference in the weight gain of the animals in the administration group within 14 days ( image 3 ), the animal was in good activity, good mental state, white and shiny coat, normal ur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average tumor weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com