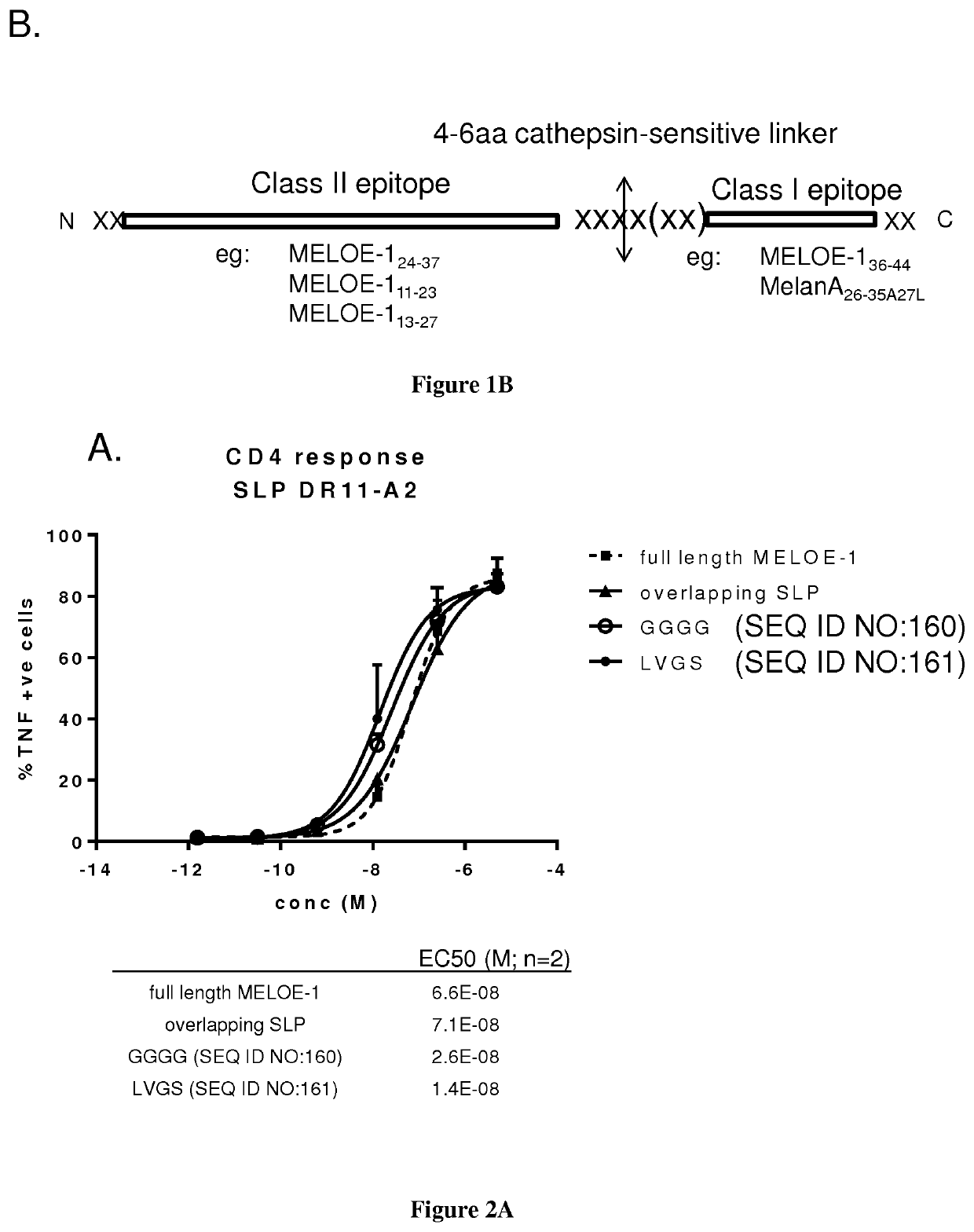
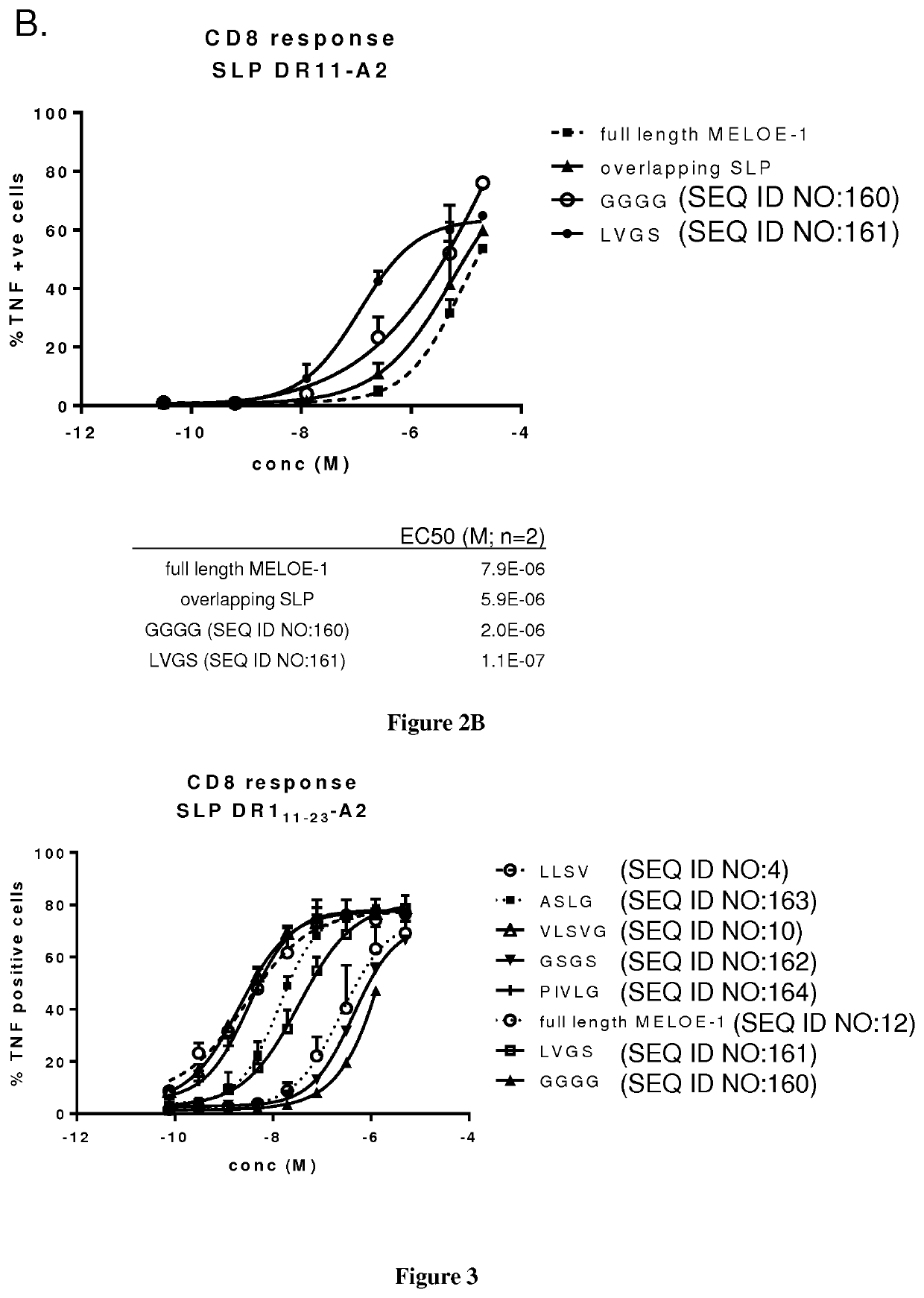
New vaccinal strategy
a new vaccinal strategy and vaccine technology, applied in the field of new vaccinal strategies, can solve the problems of limited induction of effector t cells, disappointing clinical efficacy, and the inability to vaccinate with full-length recombinant proteins,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0147]Material & Methods
[0148]Peptide Synthesis
[0149]Designed peptides were purchased at >90% purity (Protogenix, Genecust). Lyophilised powder was resuspended at 10 mMstock in DMSO and stored at −80° C. Purity was assessed by HPLC and precise quantification was calculated based on protein content analysis.
[0150]Mice
[0151]HLA-DRB1*0101 / HLA-A*0201 transgenic mice (A2 / DR1 mice) have been previously described (Pajot et al Eur. J. Immunol 2004, Dosset et al Clin Cancer Res, 2012, Rangan et al Oncotarget, 2017). Mice were bred and housed at Animalerie centrale UFC / UFR “SMP” Besançon. Female mice 6-10 weeks old were used in the experiments. All experiments were performed according to the good laboratory practices after agreement #58 by the local ethical committee.
[0152]Tumor Cell Line
[0153]The SARC-L1 cell line which spontaneously occurred in A2 / DR1 mice was genetically modified to over-express HLA-A*0201 as previously described (Rangan et al Oncotarget, 2017). SARC-A2 cells were then tra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com