Urea derivatives for treating and/or preventing cancer
A cancer, compound technology, applied in the medical field, can solve problems such as cancer that cannot be finally cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0311] Example A: Chemistry
[0312] 1. General Information
[0313] Methanol, ethyl acetate, diethyl ether and dichloromethane were purchased from Carlo Erba. Anhydrous DMF (99.8%, stored under septum) was purchased from Sigma Aldrich. All chemicals were purchased from Aldrich, Fisher or Alfa Aesar. Thin layer chromatography (TLC) was performed on pre-coated Merck 60GF254 silica gel plates and visualized first by visualization under UV light (254nm and 360nm). 1 H and 13 C NMR spectra were recorded on a Bruker Advance 200 MHz spectrometer or a Bruker Advance 400 MHz or Bruker Advance 500 MHz. Mass spectra (ESI-MS) were recorded on a Bruker (Daltonics Esquire 3000+). HRMS spectra were recorded at m / z 200 at a resolution of 140 000 on a ThermoFisher QExactive (ESI-MS). The purity of the compound was further determined by HPLC analysis on a JASCO PU-2089 instrument using the following method:
[0314] - Method 1: Phenomenex Jupiter C18, 5 μm 250x 300mm 300A. UV detec...
Embodiment B
[0355] Example B: Biology
[0356] 1. Materials and Methods
[0357] Reagents and Antibodies
[0358] Sunitinib, SB203580, SB225002, cisplatin and danirisin were purchased from Selleckchem (Houston, USA). Anti-HSP60 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-AKT, anti-phospho-AKT, anti-ERK, anti-phospho-ERK antibodies were from Cell Signaling Technology (Beverly, MA, USA).
[0359] cell culture
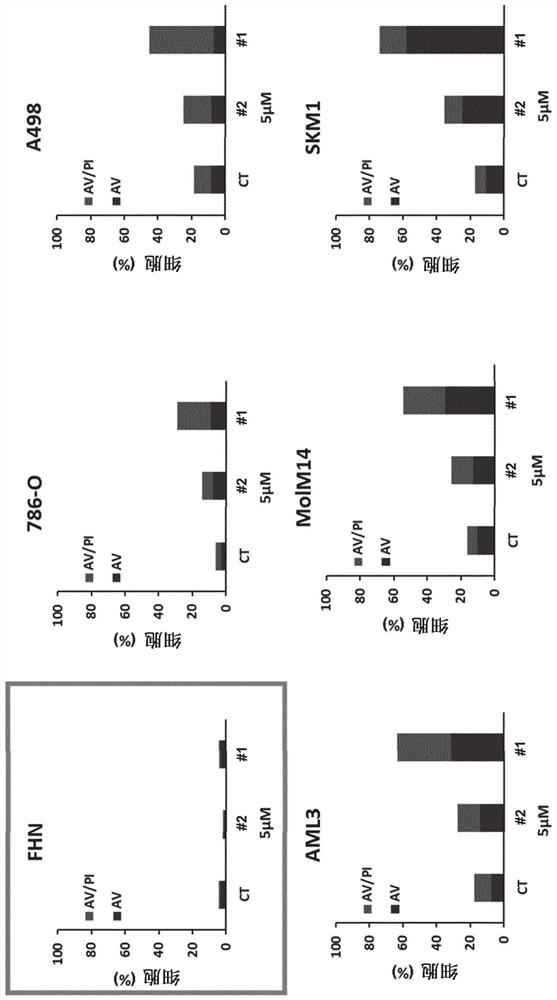
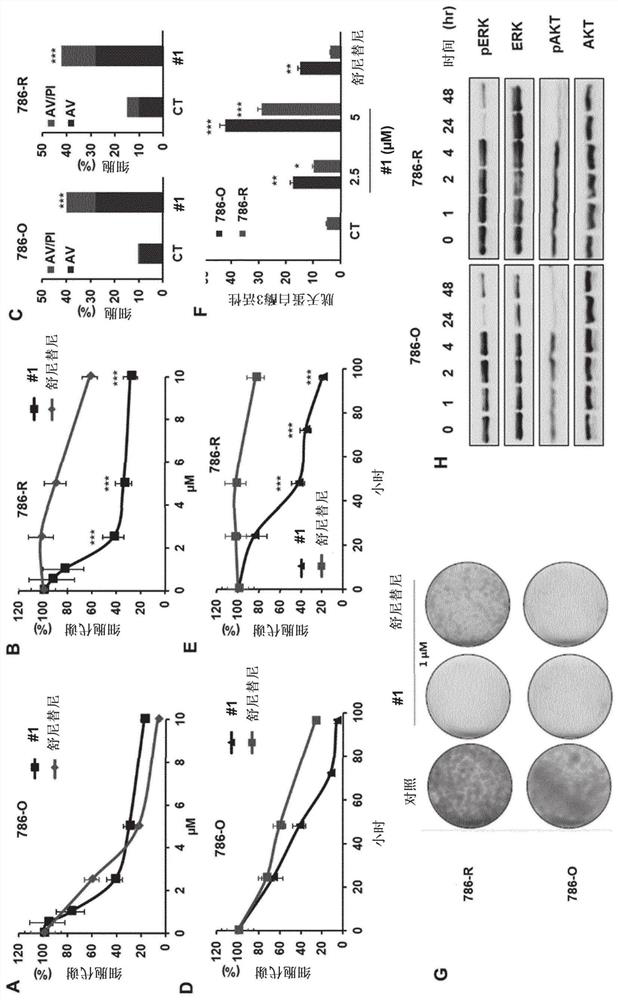
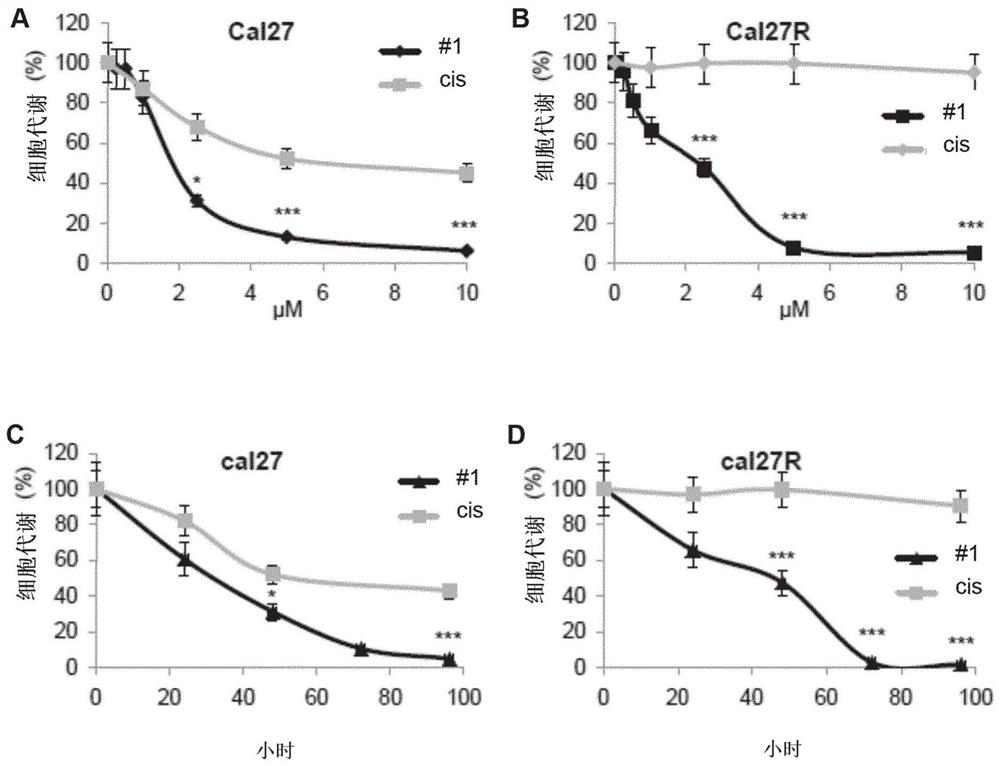
[0360] RCC4, 786-0 (786) and A498 (498) RCC cell lines, MDA-MD-231 breast cell line, Cal27 and Cal33 head and neck cell lines were purchased from American Tissue Culture Collection (ATTC). Resistant cells 786R (resistance to sunitinib), CAL27RR (resistance to photon multiple irradiation and cisplatin) and CAL33RR (photon multiple irradiation and cisplatin resistance) were provided by the present inventors. OCI-AML2, OCI-AML3, Molm13 and Molm14 acute myeloid cell line (AML), and K562 chronic myeloid cell line (CML), SKM1 myelodysplastic ce...
Embodiment C
[0416] Example C: Medulloblastoma
[0417] 1. Materials and Methods
[0418] cell culture
[0419] DAOY and HD-MBO3 cell lines were purchased from ATCC. They were in the incubator with 10% fetal bovine serum (D.Dutscher) and 1mM sodium pyruvate ( MEMα(1X)+Glutamax from Life Technologies) Incubate together at 37°C.
[0420] Cell viability (XTT)
[0421] 5000 DAOY cells and 50000 HD-MB03 cells were incubated in 96-well plates with different inhibitor concentrations for 24 hours and 48 hours. A control without cells was performed. Add 50 µl of 3'-[1-phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonate sodium hydrate (XTT) reagent into each hole. The assay is based on the formation of the orange formazan dye by lysis of the yellow tetrazolium salt XTT by metabolically active cells. The absorbance of the formazan product, reflecting cell viability, was measured at 450 nm. Each assay was performed in triplicate.
[0422] proliferation test
[04...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com