Nattokinase external transdermal absorbent and preparation method thereof
The technology of a transdermal absorbent and nattokinase is applied in the field of nattokinase external transdermal absorbent and its preparation, which can solve the problems of poor transdermal property, poor stability and low bioavailability of nattokinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Preparation and Drug Release Test of Nattokinase External Transdermal Absorbent
[0048] 1. Preparation of Nattokinase External Transdermal Absorbent
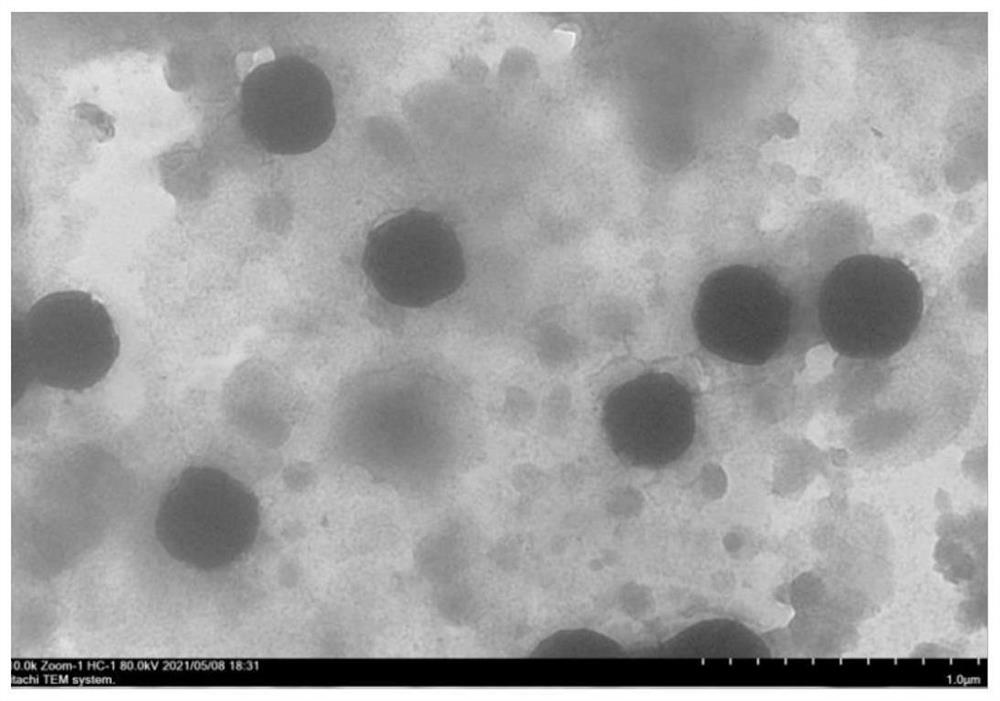
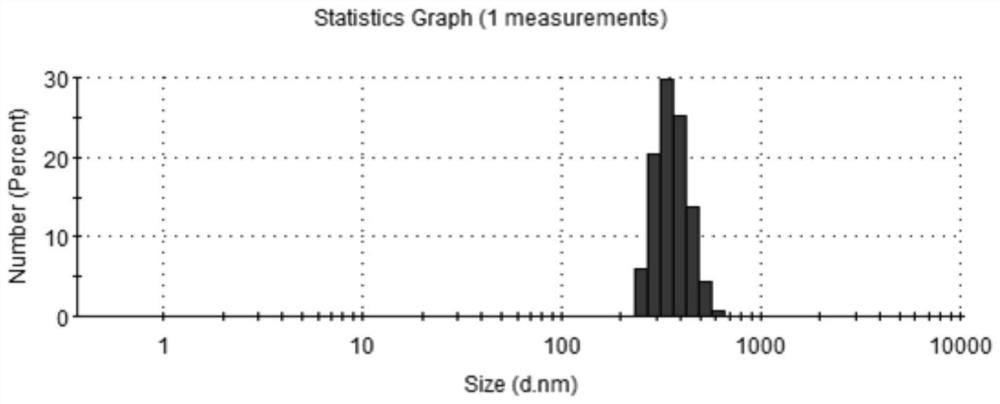
[0049] (1) Preparation of Nattokinase Molecular Microcapsules
[0050] Prescription: nattokinase: soybean lecithin: sodium deoxycholate (w / w / w)=1:5:0.5;
[0051]Preparation steps: Dissolve 0.26g of nattokinase dry powder in 20mL of normal saline as the water phase; dissolve 1.3g of soybean lecithin and 0.13g of sodium deoxycholate in 30mL of absolute ethanol, dissolve evenly, and use it as the organic phase; Evaporate in a rotary evaporator to form a uniform film, add the water phase, continue to hydrate for 60 minutes, and form a suspension of microcapsules. Ultrasonic (ultrasonic power: 200W) is dispersed in an ice bath for 5 minutes, and refrigerated and centrifuged at 4°C (centrifugal speed (15000rpm) for 20min, discard the supernatant, collect the precipitate, freeze-dry to obtain nattokinase molecula...
Embodiment 2
[0066] The preparation (three batches) of embodiment 2 nattokinase external transdermal absorption agent pilot product
[0067] According to the method of Example 1, the prescription in Table 2 was prepared into three batches of pilot product of nattokinase external transdermal absorption agent, 100 parts / batch, and the dosage range of nattokinase was guaranteed to be 0.250g~0.283g / per part.
[0068] Table 2
[0069]
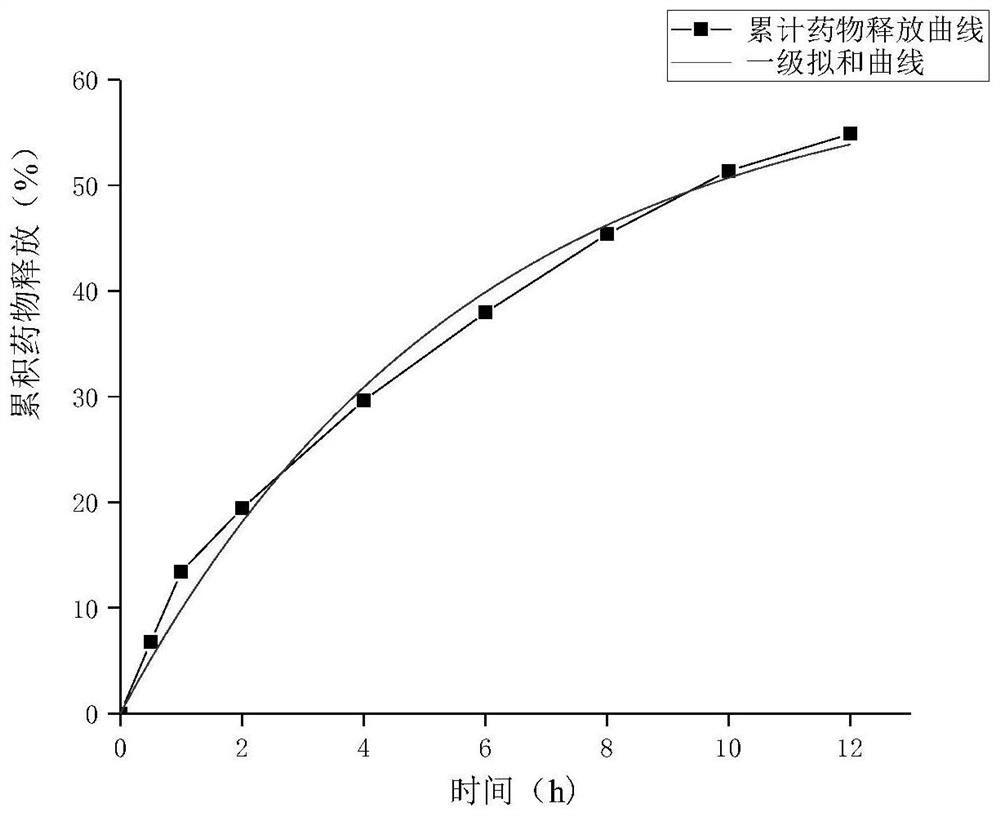
[0070] Pilot-scale product (three batches) nattokinase transdermal absorber release assay mode is the same as implementation example 1, and detection result is as shown in table 3, Figure 4 Nattokinase release curves are shown.
[0071] table 3
[0072]
[0073]
[0074] Depend on Figure 4 As can be seen from Table 3, the above-mentioned pilot product of nattokinase external transdermal absorption agent is consistent with the release rule of nattokinase described in Example 1. Nattokinase reaches the effective concentration in the body after 2 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com