A kind of reducing molten salt medium and preparation method thereof
A molten salt medium and molten salt technology, applied in the field of reducing molten salt medium and its preparation, can solve the problems of long production cycle, high process energy consumption, and high cost, and achieve the effects of reducing power consumption, avoiding reverse reactions, and reducing volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation of embodiment 1 reducing molten salt medium
[0075] In this example, mixed salt NaOH-Na 2 CO 3 -NaCl as raw material, with mixture NaCl-CaCl 2 It is molten salt medium flux; the mixed salt NaOH-Na 2 CO 3 -NaOH, Na in NaCl 2 CO 3 And the mass ratio of NaCl is 2:1:1. Mixture NaCl-CaCl 2 Medium NaCl and CaCl 2 The mass ratio is 1:2.
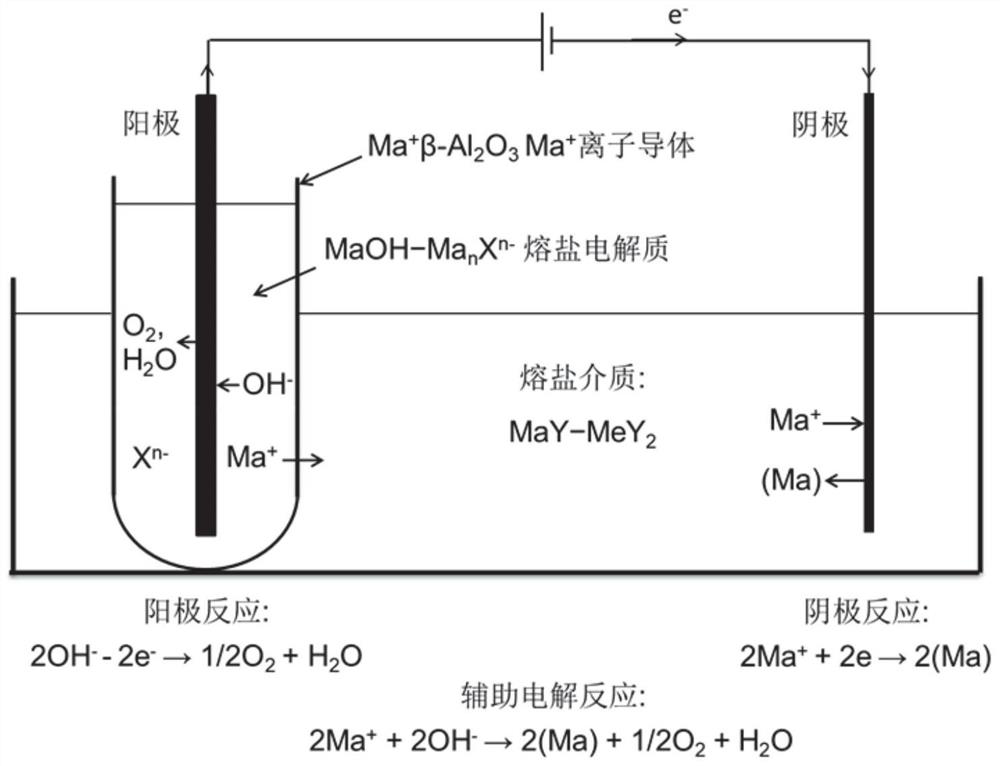
[0076] Such as figure 1 As shown, (1) will be filled with NaOH-Na 2 CO 3 - Na of NaCl raw material + β-Al 2 o 3 Container into NaCl-CaCl 2 In molten salt medium, Na + β-Al 2 o 3 The outside of the container is directly connected with NaCl-CaCl 2 The molten salt medium is in contact with each other, and makes NaOH-Na 2 CO3 -NaCl molten salt and molten NaCl-CaCl 2 The molten salt medium flux is completely isolated; (2) when Na + β-Al 2 o 3 The raw materials in the container are completely melted to form molten NaOH-Na 2 CO 3 -After the NaCl molten salt, an electronic conductor iron rod is inserted in t...
Embodiment 2
[0078] The preparation of embodiment 2 reducing molten salt medium
[0079] In this example, mixed salt KOH-K 2 CO 3 -KCl as raw material, with mixture KCl-CaCl 2 It is molten salt medium flux; the mixed salt KOH-K 2 CO 3 - KOH, K in KCl 2 CO 3 And the mass ratio of KCl is 3:1:1. Mixture KCl-CaCl 2 Medium KCl and CaCl 2 The mass ratio is 2:1.
[0080] Such as figure 1 As shown, (1) KOH-K 2 CO 3 -KCl melts to form KOH-K 2 CO 3 - KCl molten salt, put in K + β-Al 2 o 3 In container; K + β-Al 2 o 3 The outside of the container is directly mixed with KCl-CaCl 2 The molten salt medium is in contact with the flux, making KOH-K 2 CO 3 -KCl molten salt and molten KCl-KCl 2 The molten salt medium flux is completely isolated; (2) in KOH-K 2 CO 3 -In the KCl molten salt, the electron Ni conductor rod is inserted as an inert anode; in KCl-KCl 2 A stainless steel electronic conductor rod is inserted into the molten salt medium flux as an inert cathode; it constitut...
Embodiment 3
[0081] The preparation of embodiment 3 reducing molten salt medium
[0082] In this embodiment, mixed salt LiOH-Li 2 CO 3 -LiCl as raw material, with mixture LiCl-CaCl 2 It is molten salt medium flux; the mixed salt LiOH-Li 2 CO 3 -LiOH, Li in LiCl 2 CO 3 And the mass ratio of LiCl is 3:1:2. Mixture LiCl-CaCl 2 Medium LiCl and CaCl 2 The mass ratio is 1:1.75.
[0083] Such as figure 1 As shown, (1) the LiOH-Li 2 CO 3 -LiCl melts to form LiOH-Li 2 CO 3 - LiCl molten salt, put Li + β-Al 2 o 3 In the container; Li + β-Al 2 o 3 The outside of the container is directly connected with LiCl-CaCl 2 The molten salt medium is in contact with the flux, making LiOH-Li 2 C0 3 -LiCl molten salt and molten LiCl-LiCl 2 The molten salt medium flux is completely isolated; (2) in LiOH-Li 2 CO 3 -In the LiCl molten salt, an electronic conductor iron rod is inserted as an inert anode; in LiCl-LiCl 2 A stainless steel electronic conductor rod is inserted into the molten sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com