Humanized monoclonal antibody of novel coronavirus and application thereof
A technology of cloning antibodies and source list, which is applied in the field of medicine, can solve the problems of no specific drugs being approved for marketing, and achieve the effect of good neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
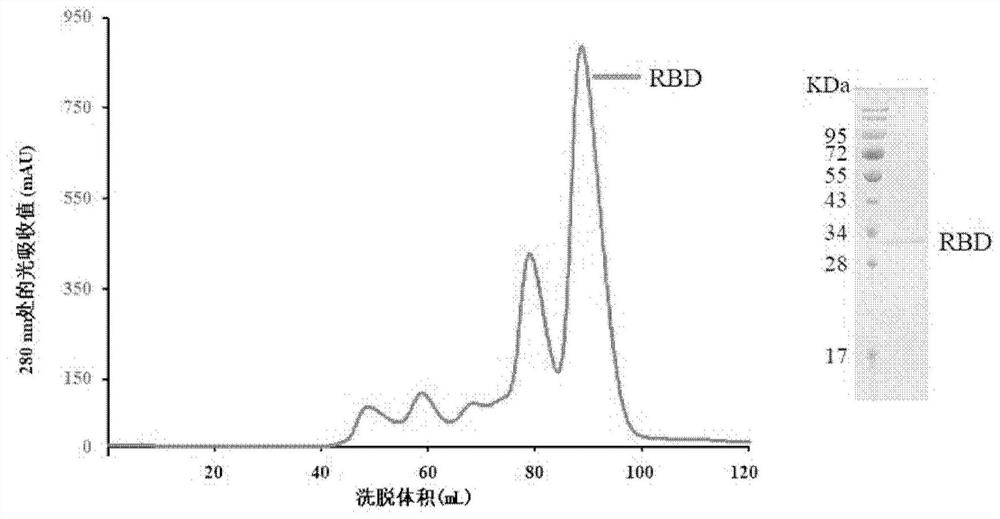
[0052] Example 1: Expression and purification of 2019-nCoV RBD
[0053] At the 3' end of the coding region of the 2019-nCoV RBD protein (the amino acid sequence is shown in SEQ ID NO: 9), connect the coding sequence of 6 histidine tags (hexa-His-tag) and the translation stop codon, and connect EcoRI and XhoI were constructed into pFastBacl vector (purchased from Invitrogen). The ligated product was then transformed into DH10Bac competent cells (purchased from Tiangen) for baculovirus recombination. The recombinant baculovirus was extracted, transfected into sf9 cells (purchased from Invitrogen) for packaging of the baculovirus, and then amplified by the virus, added to Hi5 cells (purchased from Invitrogen) for 2019-nCoV RBD protein synthesis Express.
[0054] After the cell culture solution containing the target protein is purified by nickel ion affinity chromatography (HisTrap TMHP (GE)) and gel filtration chromatography (SuperoseTM 6 Increase10 / 300GL (GE)), a relatively pu...
Embodiment 2
[0055] Example 2: Isolation of 2019-nCoV RBD protein-specific memory B cells
[0056] With the informed consent of the discharged personnel after 2019-nCoV RBD infection, 15 mL of blood was collected to separate PBMCs. Separated PBMCs in 10 7 The density and final concentration of 400nM 2019-nCoV RBD protein was incubated on ice for half an hour, then washed twice with PBS, and then incubated with the following antibodies (both purchased from BD): anti-human CD3 / PE-Cy5, anti-human CD16 / PE-Cy5, anti-human CD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-human CD38 / APC, anti-human IgG / FITC, and anti-His / PE. After antibody incubation on ice for half an hour, PBMCs were washed twice with PBS.
[0057] After washing with PBS, PBMCs were sorted by FACSAria III to collect PE-Cy5-APC-APC-Cy7+Pacific Blue+FITC+PE+ cells (i.e. B cells), and directly collected them into 96-well plates, 1 cell / well .
Embodiment 3
[0058] Example 3: Single B cell PCR, sequence analysis and human antibody design
[0059] According to the method described in Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus published in Science Translational Medicine, Volume 8, No. 369 in December 2016 by Qihui Wang et al., the B cells obtained in Example 2 were Reverse transcription was carried out by Superscript III reverse transcriptase (Invitrogen). The reverse transcription primers were listed in Table 1 and reacted at 55°C for 60 minutes.
[0060] Table 1. Primers for reverse transcription reactions
[0061]
[0062] Using this reverse transcription product as a template, PCR was performed with HotStar Tap Plus enzyme (QIAgen) to amplify the antibody variable region sequence (PCRa). Corresponding primers were designed, and the reaction conditions were as follows: 95°C, 5min; 95°C for 30s, 55°C (heavy chain / κ chain) for 30s, 72°C for 90s, 35 cycles; 72°C, 7min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com