Application of kaurane compound in preparation of medicine for preventing and treating inflammatory bowel disease
A technology for inflammatory bowel disease and kauri, which is applied to the application field of kauri compounds in the preparation of medicines for preventing and treating inflammatory bowel disease, and can solve the problems of patient infection, unreported, easy to fail and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
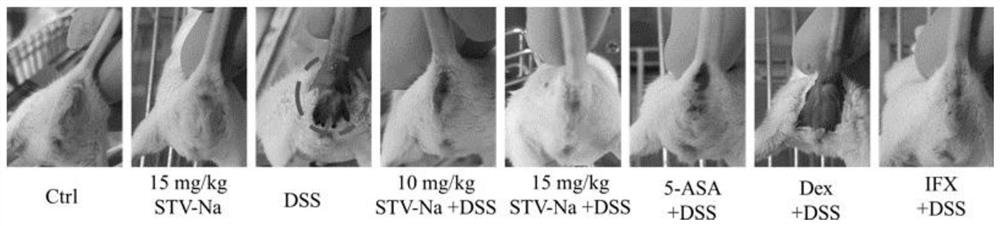
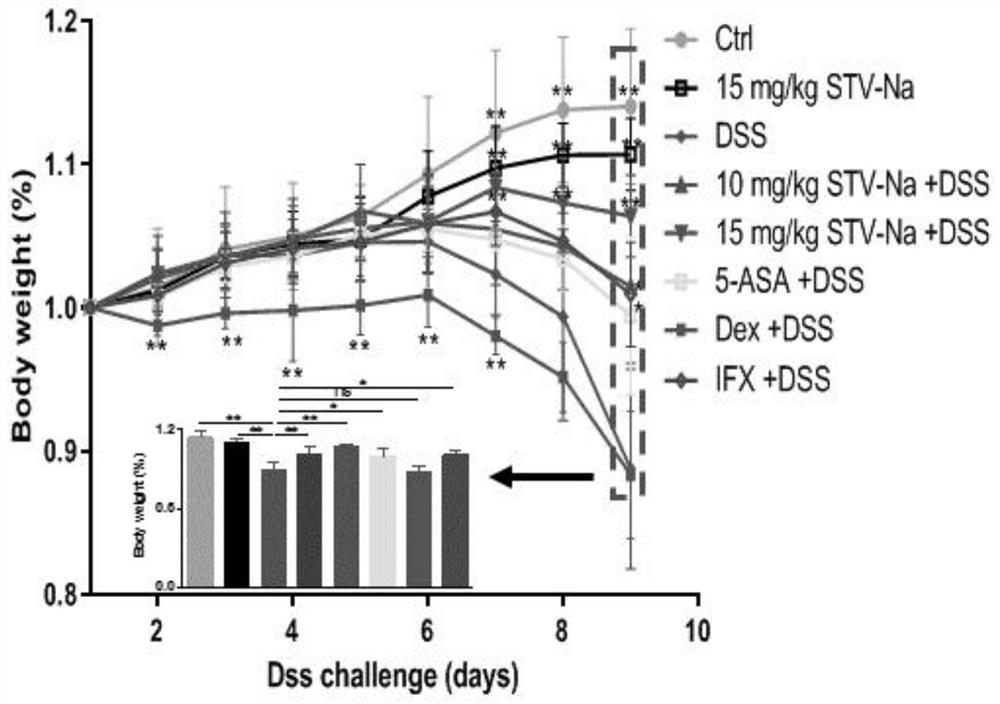
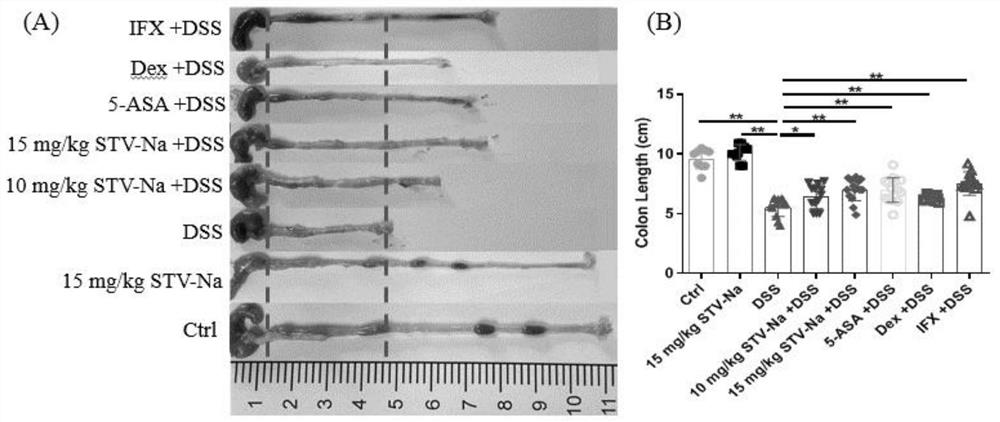
[0061] This case mainly observes the effect of intraperitoneal injection of different doses of compound A on dextran sodium sulfate (DSS)-induced ulcerative colitis in mice.
[0062] Clean grade Balb / c mice, 6-8 weeks old, male. Divide into eight groups at random, establish normal group (give normal saline), model group (free drinking 3.5%DSS), compound A (5mg / kg) blank group, compound A (10mg / kg)+DSS group, compound A (15mg / kg)+DSS group, positive control group 5-ASA(50mg / kg)+DSS group, positive control group Dex(10mg / kg)+DSS group, positive control group IFX(1mg / kg)+DSS group, each group 15 only. After grouping, the mice in the experimental group were intraperitoneally injected with compound A, Dex and IFX, and 5-ASA, and the mice in the control group were injected with normal saline intraperitoneally. The experimental group was administered for 2 consecutive days. From the third day, both the experimental group and the control group began to drink 3.5% DSS aqueous solutio...
Embodiment 2
[0069] This case mainly observes the permeability of the colon of experimental mice in each group.
[0070] 1) Principle: The permeability of animal intestine can be semi-quantitatively detected by using fluorescent tracer to detect the fluorescence intensity in serum.
[0071] 2) Preparation of standard curve:
[0072] Take a few normal mice, collect hemolysis-free serum, weigh 200 μg of FITC-dextran powder, dissolve it in 5ml serum, dilute it in multiples, and then use a microplate reader to detect the fluorescence intensity to obtain a standard curve.
[0073] 3) On the day of sacrificing the animals, fast 4 hours in advance.
[0074] 4) Oral administration of the prepared FITC-dextran tracer at a dose of 60 mg / kg body weight.
[0075] 5) Blood was collected from animals before sacrifice, and serum without hemolysis was collected.
[0076] 6) Serum was added to a 96-well plate, and 100 μl per well was used to detect the fluorescence intensity with a microplate reader (ex...
Embodiment 3
[0080] This case mainly illustrates the effect of compound A on the blood routine of inflammatory bowel disease.
[0081] After the 9th day, blood was collected from the mice, gently dropped into the prepared EP tubes prepared with EDTA salt for anticoagulation, and the 80-100 μL blood samples were mixed evenly, and the blood routine was tested using an automatic blood analyzer, including red blood cell count ( RBC), monocytes (MONO), neutrophils (LYMPH), platelets (PLT), hemoglobin (HGB), hematocrit (HCT) and white blood cell count (WBC), etc.
[0082] Such as Figure 8 As shown, compared with the normal group, the WBC, NEUT, LYMPH and MONO of the model group were significantly increased. After the intervention of compound A, 5-ASA and Infliximab, the WBC, NEUT, LYMPH and MONO all decreased, but the Dex group and the model group There was no significant difference in group comparison.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com