Method for synthesizing DNA coded sulfone compound through on-DNA free radical reaction promoted by visible light
A synthesis method and compound technology, applied in the field of synthesis of DNA-encoded sulfone compounds, can solve problems affecting the universality of reaction substrates and DNA screening results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
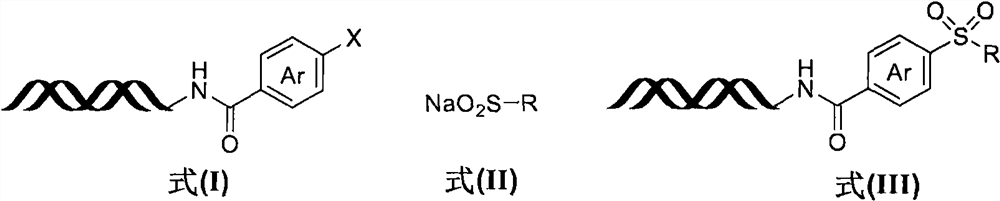
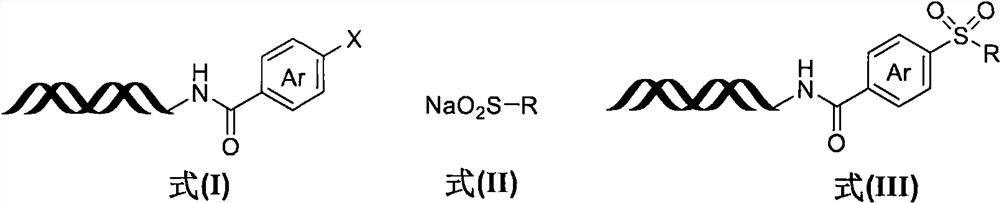
[0028] Step 1: Synthesis of DNA-encoded halogenated aromatic compound (I)
[0029]
[0030] DNA-NH 2 (4μmol), DMTMM (1M, 3mL), 3-iodo-1H-indole-6-carboxylic acid (1M, 0.4mL) were added to borax buffer solution (pH=9.5, 100mM, 3mL), and reacted at room temperature for 2h, and the reaction After completion, ethanol (15 mL) and NaCl solution (5M, 0.4 mL) were added to precipitate the DNA. Add water, acetonitrile and DIPEA to the sample tube containing the obtained precipitate, and react at 70°C for 12h. After the reaction was completed, ethanol (15 mL) and NaCl solution (5 M, 0.4 mL) were added to precipitate the DNA-encoded halogenated aromatic compound (I-a). The structure of the compound was determined by LC-MS, and the yield was 81%.
[0031] Step 2: Free radical coupling reaction of DNA-encoded halogenated aromatic compound (I-a) and sodium sulfinate (II-a)
[0032]
[0033] Immobilize the DNA-encoded halogenated aromatic compound (I-a, 10nmol) on the resin, wash with...
Embodiment 2
[0035] Using the same method as in Example 1, the reaction solvent in step 2 was replaced by DMSO with DMA to obtain a DNA-encoded sulfone compound (III-a) with a yield of 79%.
Embodiment 3
[0037] Using the same method as in Example 1, the light source in the step 2 reaction was replaced by a 365 nm light source with a 455 nm light source to obtain a DNA-encoded sulfone compound (III-a) with a yield of 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com