Recombinant adeno-associated virus for efficient tissue-specific expression of RS1 protein and application
A technology for protein expression and purpose, applied in the biological field, can solve problems such as inability to achieve tissue-specific expression of target protein, limitation of therapeutic effect, and increase in the amount of AAV vector
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 expression vector construction
[0043] The present invention first optimizes the codon of the RS1 gene, and the nucleotide sequence is shown as 645-1319bp in SEQ ID NO.4. On the basis of optimizing RS1, the present invention designs four expression cassettes, the schematic diagram of the AAV virus vector genome and the cis plasmid map of AAV virus packaging are as follows Figure 1-4 shown. Full sequence synthesis of four viral packaging cis plasmid vectors.
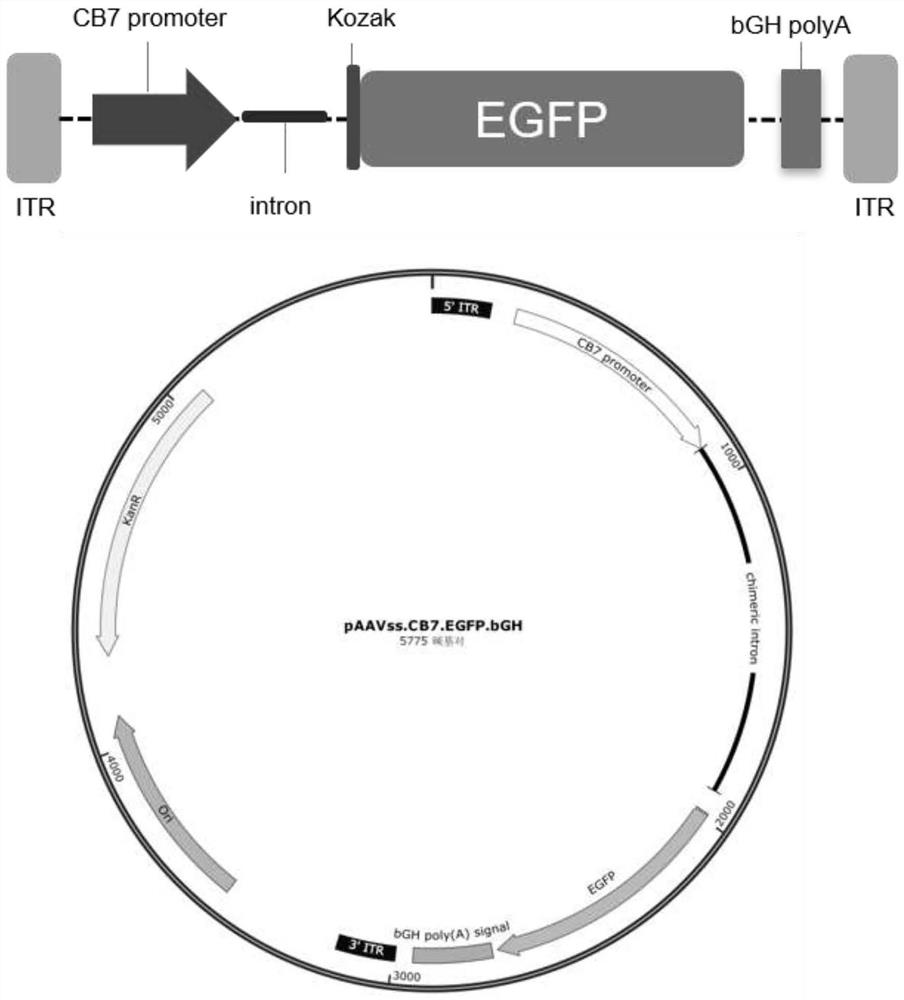
[0044] ssAAV8.CB7.EGFP.bGH viral packaging plasmid (referred to as pAAVss.CB7.EGFP.bGH), the full sequence of the plasmid is shown in SEQ ID NO.1, the main functional element site: 1-168 is the 5' inverted terminal repeat Sequence (ITR), 241-899 is the CB7 promoter sequence, 900-1916 is the intron sequence, 1982-2701 is the EGFP gene sequence, 2717-2941 is the bGHpolyA sequence, and 2992-3159 is the 3' end ITR sequence.
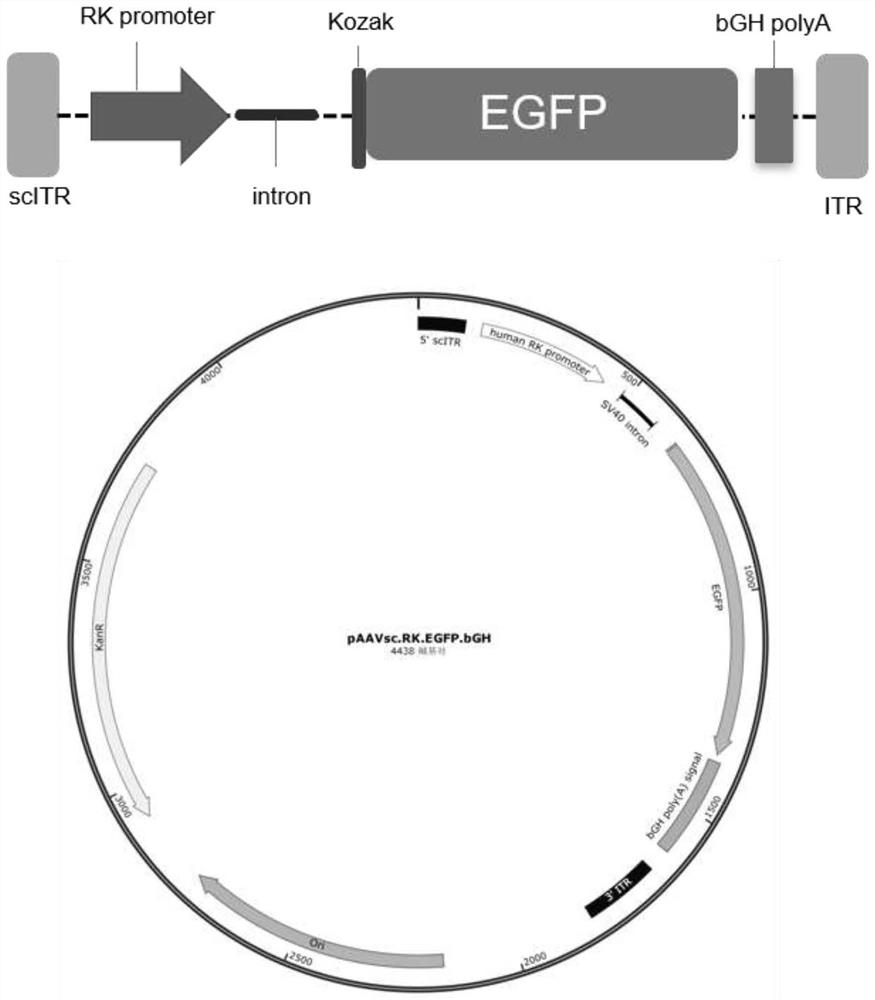
[0045] scAAV8.RK.EGFP.bGH viral packaging plasmid (pAAVsc.RK.EGFP.bGH for sh...
Embodiment 2
[0048] The specificity comparison of embodiment 2 viral packaging plasmid expressed in vitro
[0049] The pAAVss.CB7.EGFP.bGH and pAAVsc.RK.EGFP.bGH in Example 1 were transfected with the same amount of plasmids (2 μg) into HEK293 cells and optic neuroblastoma cells Y79 cultured in a six-well plate, after 48 hours The expression of EGFP was observed by immunofluorescence photography, and the results showed that pAAVss.CB7.EGFP.bGH could express EGFP in both HEK293 cells and Y79 cells, while pAAVsc.RK.EGFP.bGH could only express EGFP in Y79 cells. It shows that the RK promoter can realize tissue-specific expression of the target protein ( Figure 5 ).
Embodiment 3
[0050] Example 3 AAV virus preparation and purification
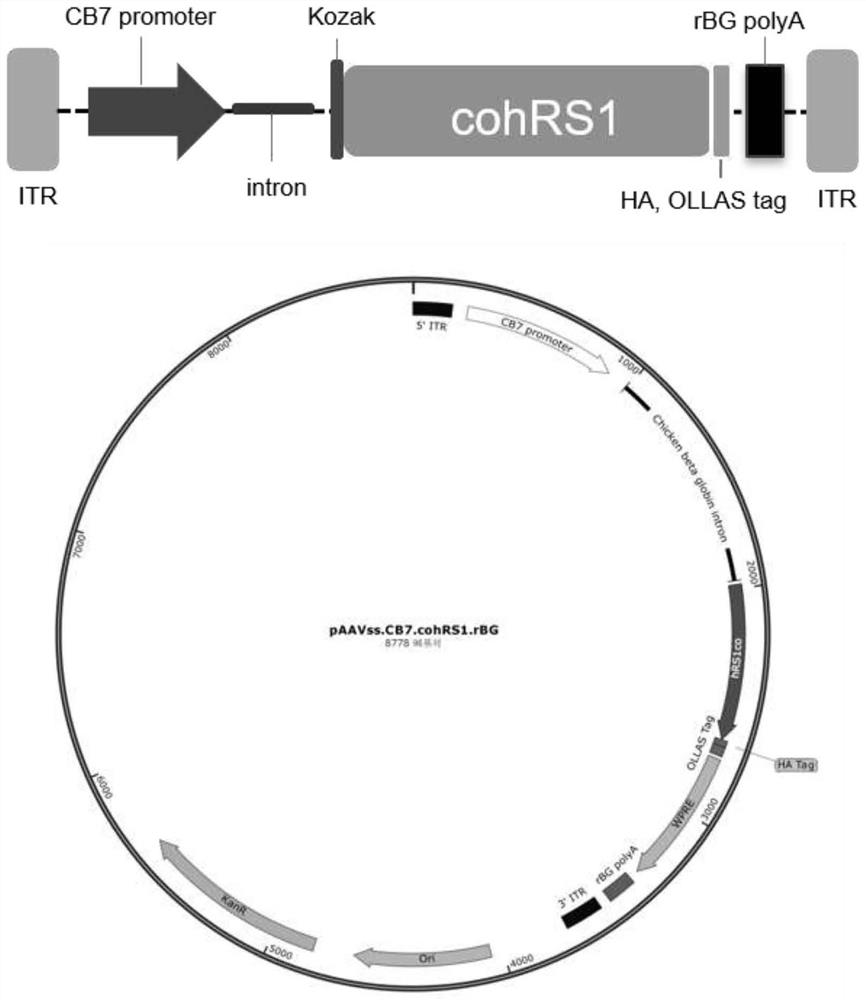
[0051] Referring to the method of packaging and purifying recombinant AAV virus reported by Martin Lock et al., the Rep of AAV2 and the Cap protein expression plasmid of AAV8 (pAAV2 / 8), helper plasmid (pAdΔF6) and AAV target gene vector cis plasmid (pAAVss. .cohRS1.rBG / pAAVsc.RK.cohRS1.bGH) were co-transfected into HEK293 cells to package and prepare virus ssAAV8.CB7.cohRS1.rBG and virus scAAV8.RK.cohRS1.bGH. After 144 hours of transfection, the cell culture supernatant was harvested and cut. After concentrating the virus liquid to the filter (TFF), use iodixanol ultrafast gradient centrifugation to purify the AAV virus, collect the purified AAV virus, use Amicon Ultra 100K centrifuge to desalt, and finally place the AAV virus in 20mM Tris (pH8.0) ,1mMMgCl 2 , 200mM NaCl and 0.001% PF68 preparations for use. The prepared AAV virus was tested for virus purity by SDS-PAGE staining, and the endotoxin content was detected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com