Preparation method of methyl tetraacetate compound
A compound, the technology of ethyl acetate, which is applied in the field of preparation of methyl tetraacetate compounds, can solve the problems of low yield and low purity, and achieve the effect of low temperature, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
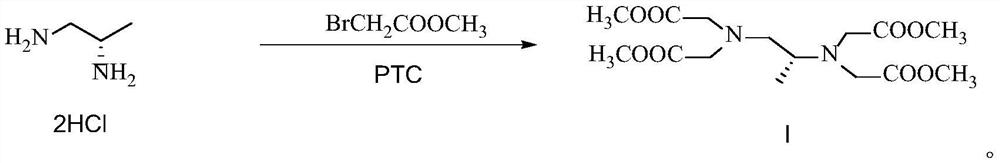
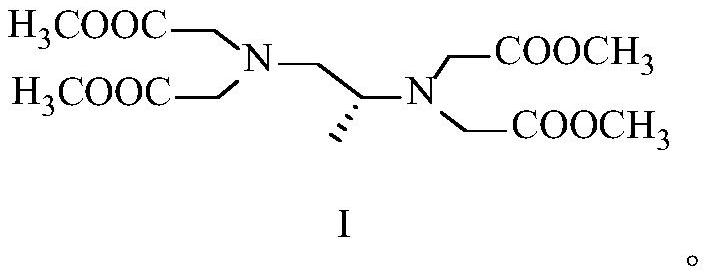
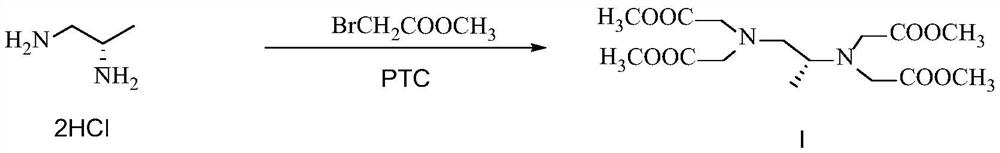
[0053] Embodiment 1 prepares formula I compound according to the method disclosed in CN104177301B
[0054] Prepare the formula I compound according to the preparation method disclosed in Example 1.8 of CN104177301B, and only increase the feeding amount from the mg level to the g level, the details are as follows:
Embodiment 11
[0056] Add (S)-1,2-diaminopropane hydrochloride (5g), methyl bromoacetate (52.02g), and potassium carbonate (46.92g) into acetonitrile (340mL), react at 50°C for 4 hours, and the reaction ends , filtered off inorganic salts, concentrated the filtrate to dryness, added HCl (150mL, 12%) solution to acidify, washed with petroleum ether, adjusted the pH to 10 with saturated sodium carbonate solution, extracted with dichloromethane, dried and concentrated to dryness, The compound of formula I was obtained with a yield of 56%.
Embodiment 12
[0058]Add (S)-1,2-diaminopropane hydrochloride (10g), methyl bromoacetate (104.04g), and potassium carbonate (93.84g) into acetonitrile (680mL), react at 50°C for 4 hours, and the reaction ends , filtered off inorganic salts, concentrated the filtrate to dryness, added HCl (300mL, 12%) solution to acidify, washed with petroleum ether, adjusted pH to 10 with saturated sodium carbonate solution, extracted with dichloromethane, dried and concentrated to dryness, The compound of formula I was obtained with a yield of 31%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com