PEG2, n-lipid derivative modified nano-carrier, preparation method and application
A lipid derivative, nanocarrier technology, applied in the field of medicine, can solve the problems of severe allergy or allergic reaction, loss of curative effect, increase of clearance rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
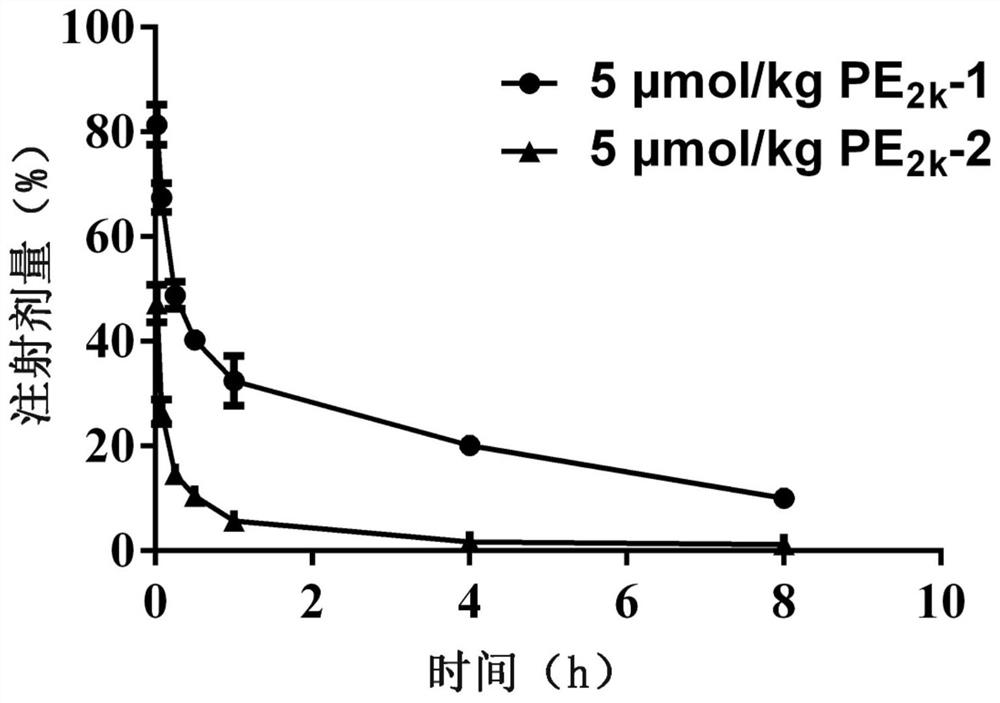
[0067] The first tail vein injection of DSPE-PEG with a total molecular weight of 2000Da 2000 DSPE-PEG with a total molecular weight of 2000Da of PEG injected into the tail vein of the modified emulsion for the second time 2000 The effect of modifying the drug-time curve of emulsion
[0068] Experimental animals:
[0069] Wistar rats (180~210g, (Provided by Lei Yunshang Pharmaceutical Laboratory Animal Center)
[0070] Dosing regimen:
[0071] Male Wistar rats, weighing 180-210 g, were randomly divided into two groups, one as the control group and the other as the experimental group, 6 rats in each group, and administered by tail vein injection. The control group was injected with 5% glucose solution for the first time, and the experimental group was injected with DSPE-PEG with a total molecular weight of 2000Da according to the phospholipid dose of 5 μmol / kg for the first time. 2000 Modifying emulsion. After the first injection, every 7 days, all groups were intravenou...
Embodiment 2
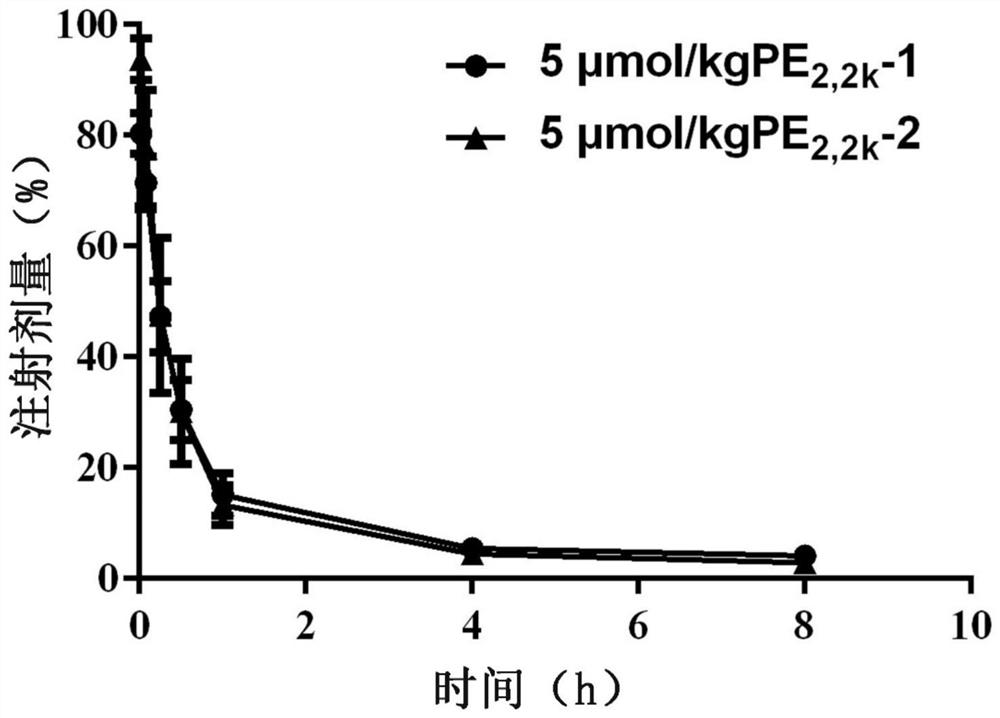
[0077] The first tail vein injection of DSPE-PEG with a total molecular weight of 2000Da 2,2k DSPE-PEG with a total molecular weight of 2000Da of PEG injected into the tail vein of the modified emulsion for the second time 2,2k The effect of modifying the drug-time curve of emulsion
[0078] Experimental animals:
[0079] Wistar rats (180-210g, ♂, provided by Leiyunshang Pharmaceutical Experimental Animal Center)
[0080] Dosing regimen:
[0081] Male Wistar rats, weighing 180-210 g, were randomly divided into two groups, and administered by tail vein injection. The control group was injected with 5% glucose solution for the first time, and the experimental group was injected with DSPE-PEG with a total molecular weight of 2000Da according to the phospholipid dose of 5 μmol / kg for the first time. 2,2k Modifying emulsion. After the first injection, every 7 days, all groups were intravenously injected with 5 μmol phospholipids / kg PEG with a total molecular weight of 2000 Da ...
Embodiment 3
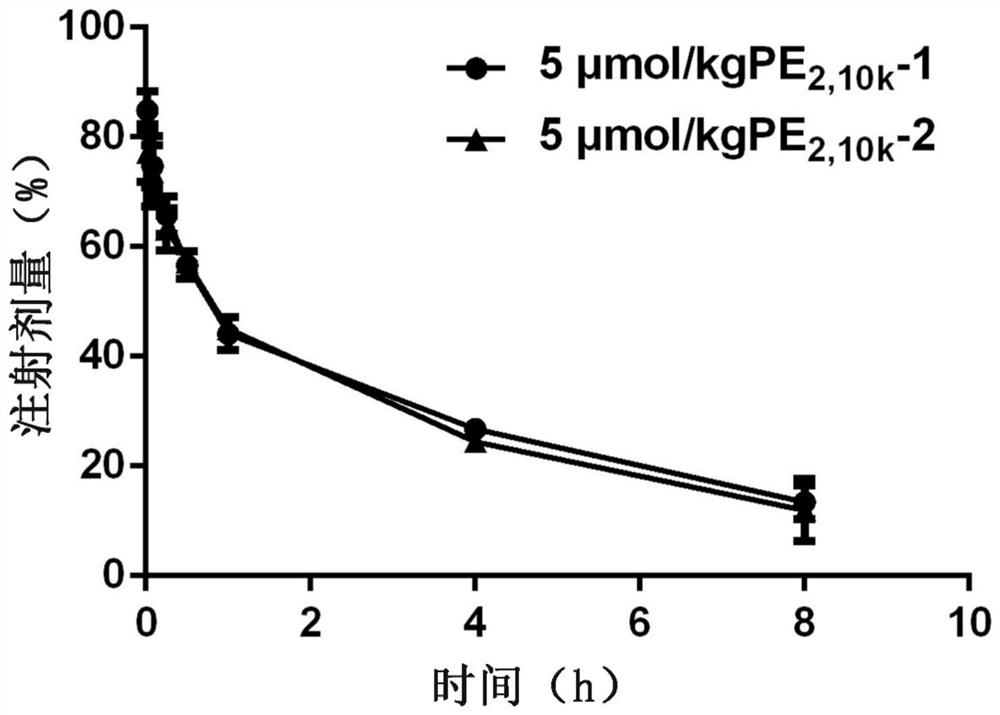
[0086] The first tail vein injection of DSPE-PEG with a total molecular weight of 10000Da 2,10k DSPE-PEG with a total molecular weight of 10000Da PEG injected into the tail vein of the modified emulsion for the second time 2,10k The effect of modifying the drug-time curve of emulsion
[0087] Experimental animals:
[0088] Wistar rats (180-210g, ♂, provided by Leiyunshang Pharmaceutical Experimental Animal Center)
[0089] Dosing regimen:
[0090] Male Wistar rats, weighing 180-210 g, were randomly divided into two groups, and administered by tail vein injection. The control group was injected with 5% glucose solution for the first time, and the experimental group was injected with DSPE-PEG with a total molecular weight of PEG of 10000Da according to the phospholipid dose of 5 μmol / kg for the first time. 2,10k Modifying emulsion. Seven days after the first injection, all groups were intravenously injected with 5 μmol phospholipids / kg of DSPE-PEG with a total molecular wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com