Magnetic resonance contrast agent as well as preparation method and application thereof
A magnetic resonance contrast agent and reaction technology, which is applied in the fields of nuclear magnetic resonance/magnetic resonance imaging contrast agents, chemical instruments and methods, and pharmaceutical formulations, and can solve problems such as huge side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
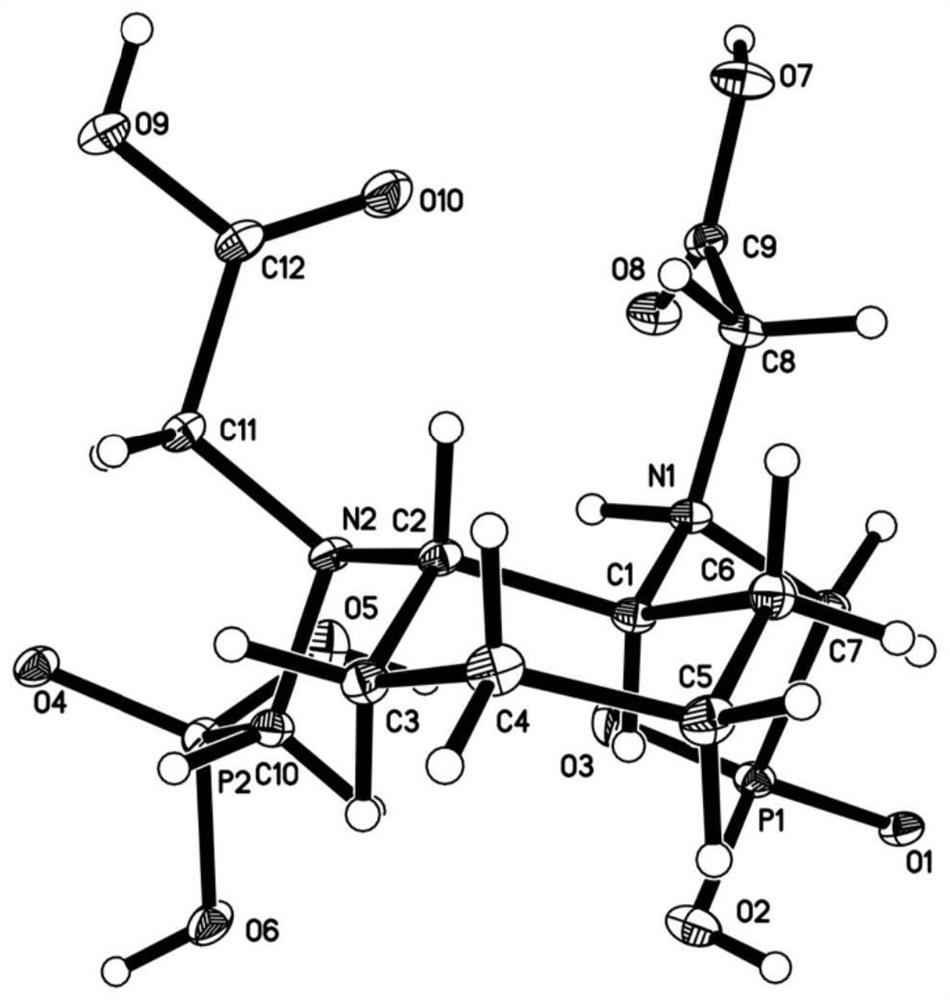
[0066] To prepare trans-cyclohexyldiamine-dimethylphosphonic acid-diacetic acid ligand, the synthetic route is as follows:
[0067]
[0068] The specific preparation method of the ligand is as follows:
[0069] (1) Preparation of compound 1
[0070] With 100mL of anhydrous tetrahydrofuran (THF) as solvent, add cyclohexanediamine (10g, 98%, 85.8mmol), diethyl phosphite (24.2g, 98%, 171.6mmol), paraformaldehyde (total amount 8.31g , 96%, 266mmol, first add about one-fifth), heat to 95 ° C, reflux, add the remaining amount of paraformaldehyde in 4 times, and reflux for 4 hours;
[0071] After the reaction was completed, the solvent was removed by rotary evaporation under reduced pressure, and about 100 mL of dichloromethane and water were added to the raffinate for extraction. Wash once with water and saturated brine (about 50mL each time), and wash the organic phase with anhydrous Na 2 SO 4 After drying, filtering, and rotary evaporation under reduced pressure to remove d...
Embodiment 2
[0092] To prepare a paramagnetic metal manganese complex magnetic resonance contrast agent, the steps are as follows:
[0093] The compound (7.19g, 17.2mmol) obtained in (4) in Example 1 was added to 60mL of pure water, manganese chloride tetrahydrate (3.33g, 16.8mmol) was added under stirring, and the pH was adjusted to 7.3 with solid sodium hydroxide ~7.4, the resulting solution was freeze-dried to a solid of 11.68 g.
[0094] MS(ESI):[M+H] - Calculated value: 470.0, measured value: 469.9; [M+3H] + Calculated value: 472.0, measured value: 472.0.
Embodiment 3
[0096] The paramagnetic metal manganese complex magnetic resonance contrast agent (code name: KBR0826) that embodiment 2 obtains carries out following performance test:
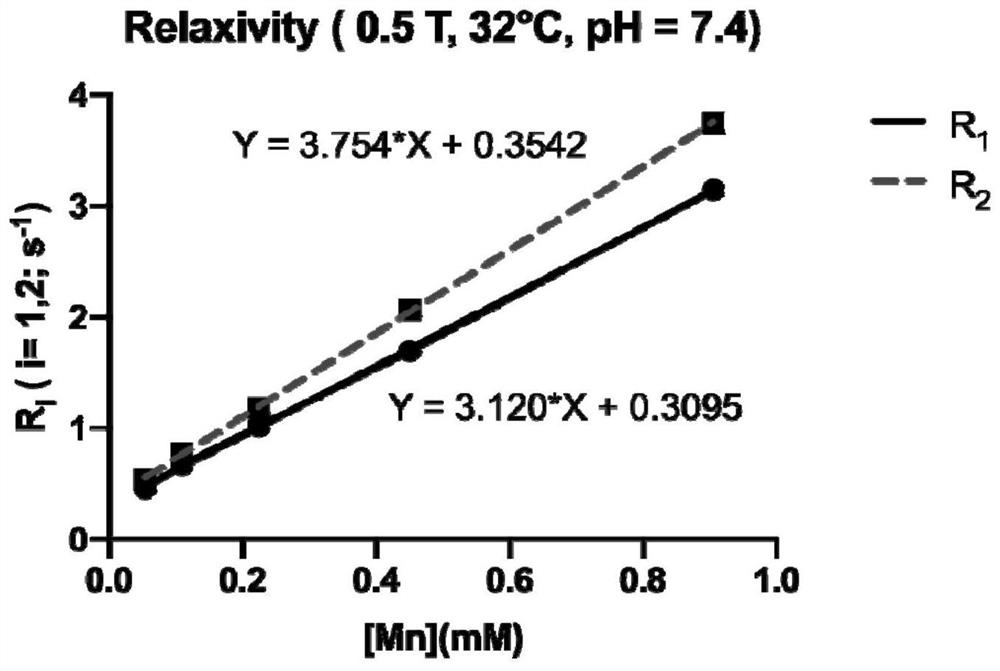
[0097] 1. Determination of relaxation efficiency
[0098] Measurement method of relaxation efficiency: configure gradient concentration contrast agent solution (0.054, 0.109, 0.224, 0.451, 0.906mM, in Hepes buffer, pH = 7.4), use Numei PQ001 NMR contrast agent relaxation rate tester (0.5±0.08 T, the main frequency of the instrument: 21.3MHz; 32°C) The T of samples with different concentrations of contrast agents was obtained by testing 1 , T 2 Relaxation time, in terms of relaxation rate R i (R i =1 / T i , i=1, 2) and contrast agent concentration for linear fitting, the slope is the relaxation efficiency (relaxivity, r i ,i=1,2). Relaxation efficiency reflects the ability of magnetic resonance contrast agents to change the relaxation time of water molecules, and is an important parameter to measure the q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com