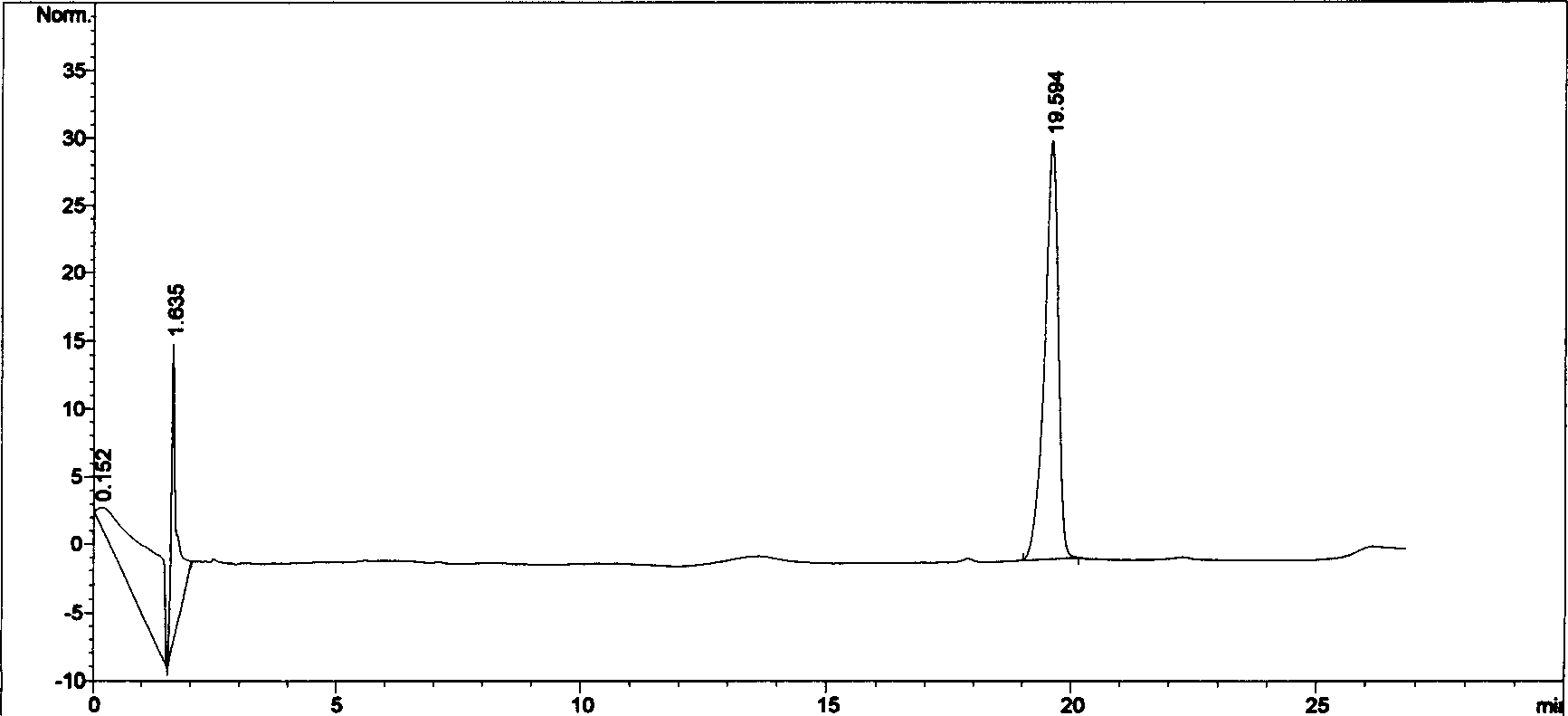
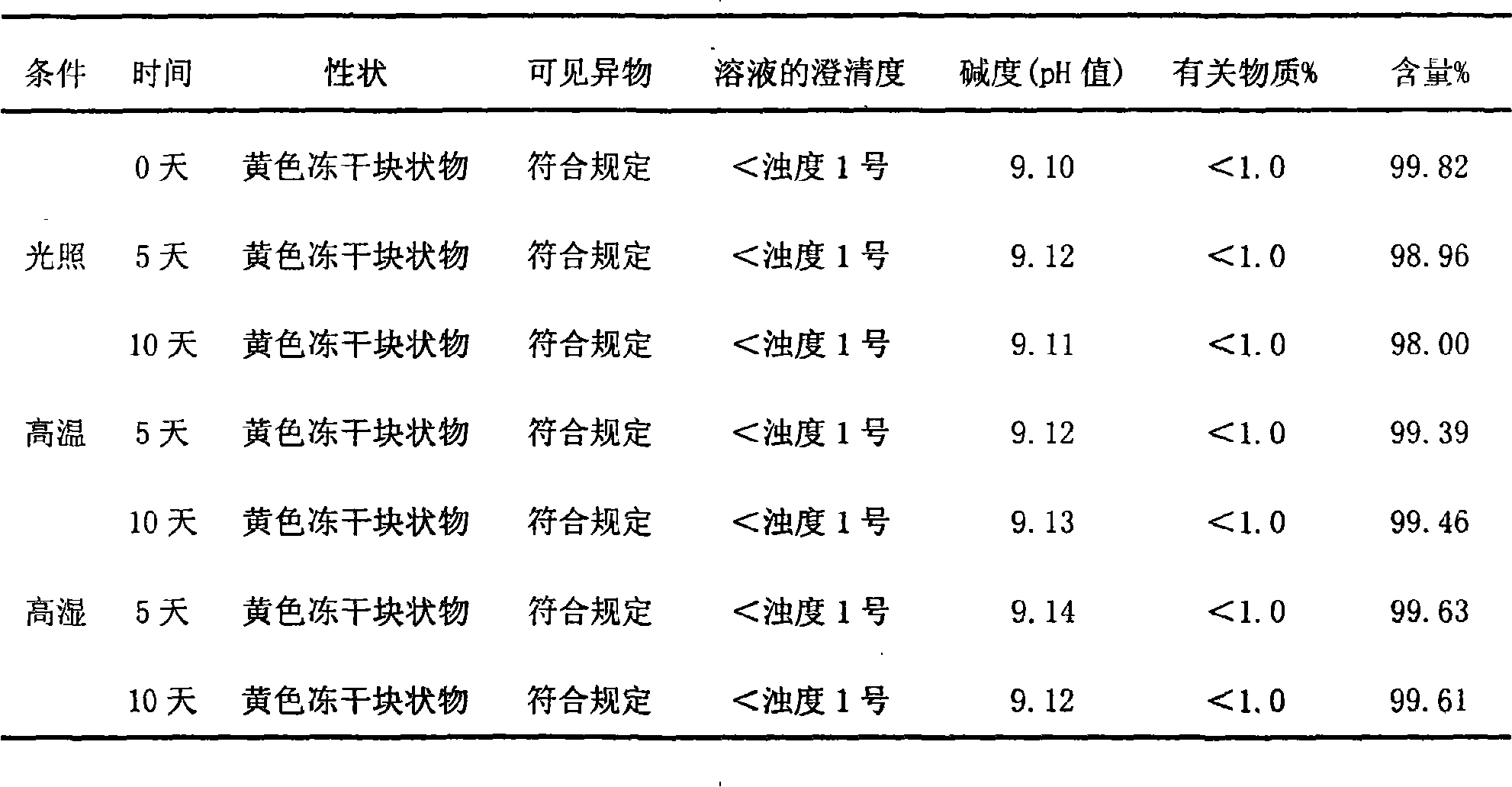
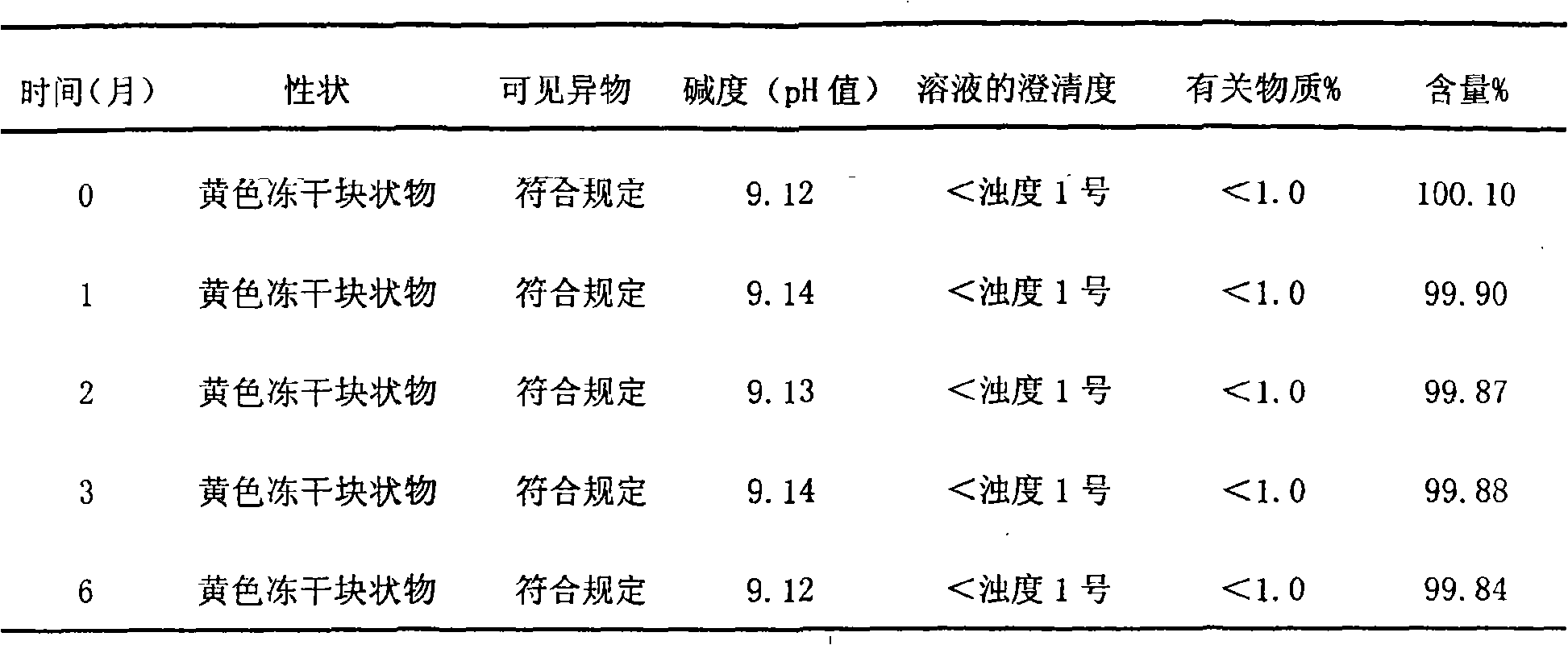
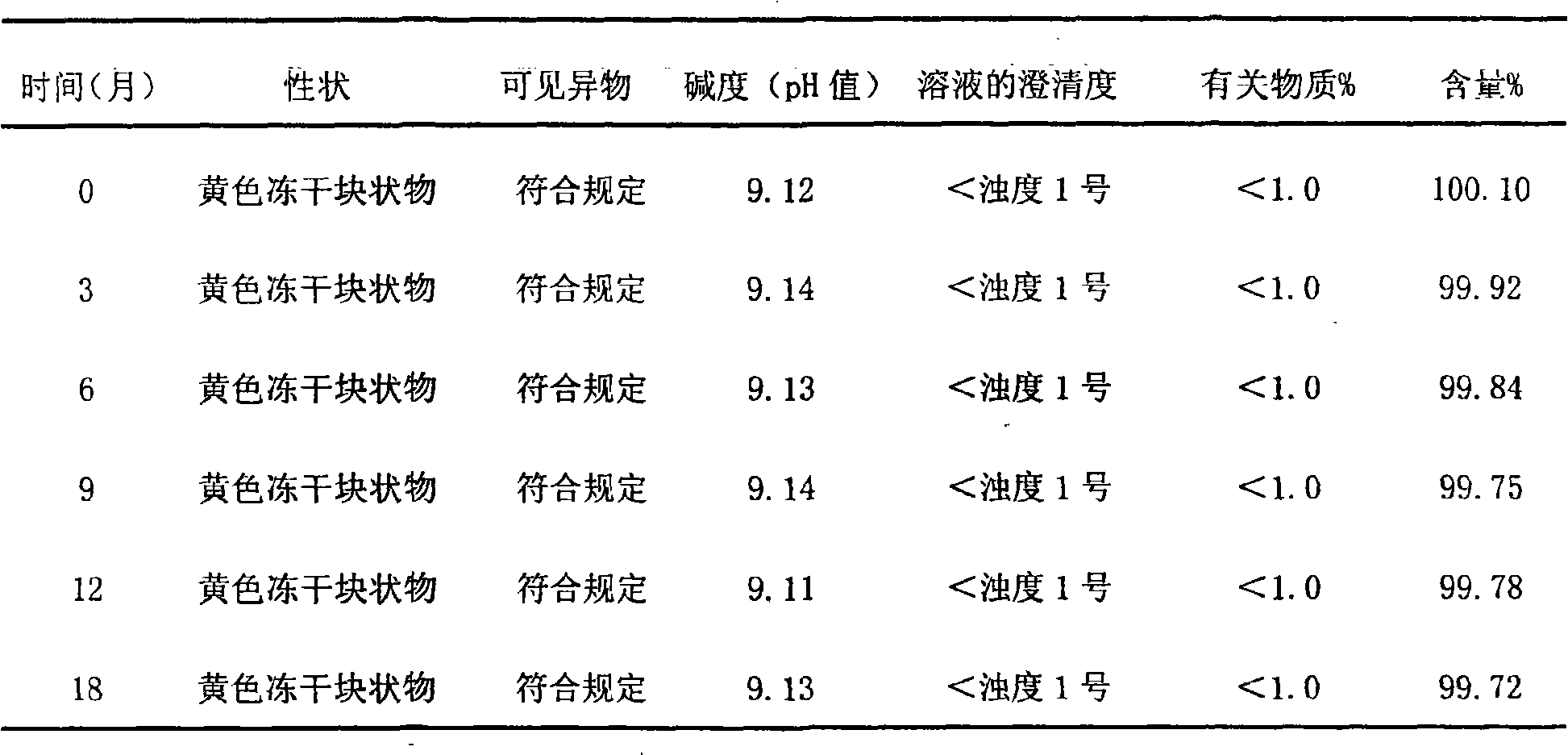
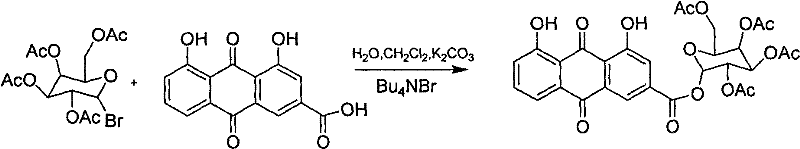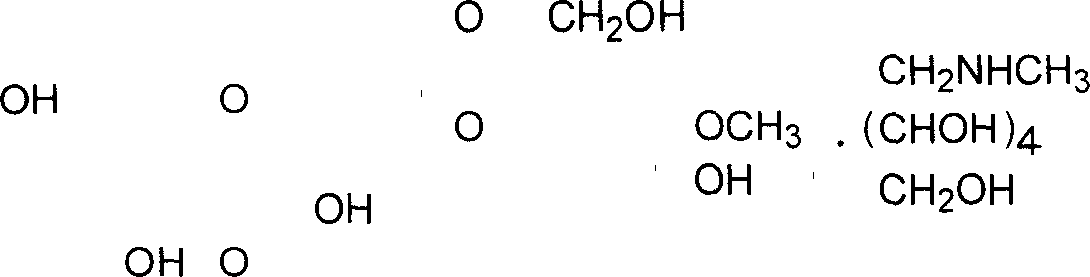Patents
Literature
47 results about "N methylglucamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
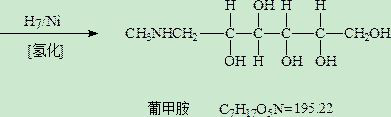
Meglumine, also known as megluminum or methylglucamine, belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six- carbon containing moeity. Meglumine is soluble (in water) and a very weakly acidic compound (based on its pKa).
Injections
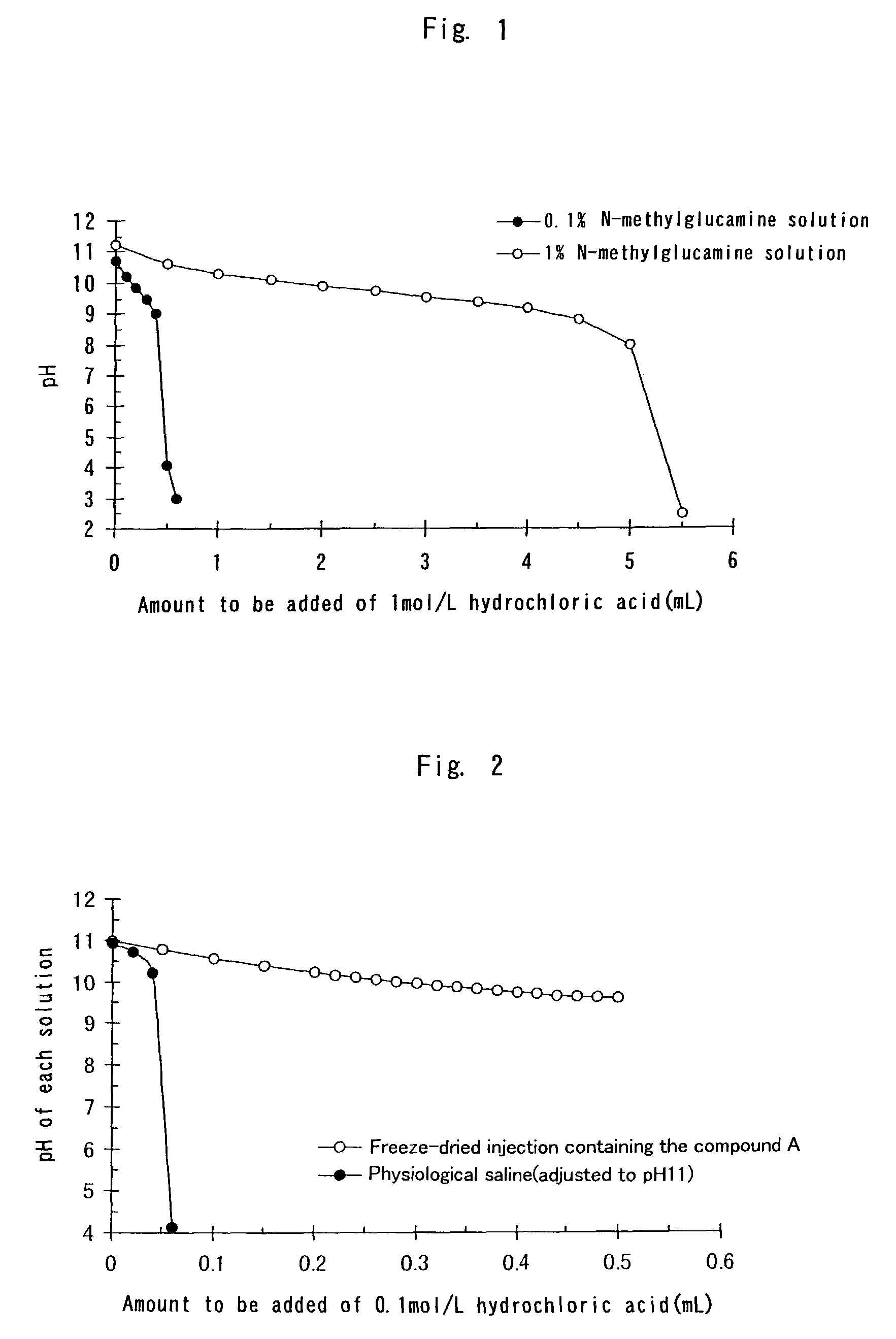
InactiveUS20030191157A1Improve stabilityReduce solubilityAntibacterial agentsPowder deliveryFreeze-dryingSolvent
An injectable composition comprises a benzimidazole compound having an antiulcer action and a strong alkali (e.g., an alkali metal hydroxide such as sodium hydroxide) in a proportion of about 1 equivalent of the latter relative to 1 mol of the former, and is substantially free from a nonaqueous solvent. The injectable composition may comprise N-methylglucamine, and a saccharide (such as mannitol). The injectable composition may be a freeze-dried preparation. The freeze-dried preparation is dissolvable in or dilutive with a distilled water for injection or an infusion solution without a nonaqueous solvent. The injectable composition is useful as an antiulcer agent.
Owner:TAKEDA PHARMA CO LTD
Injections
InactiveUS7396841B2Easy to prepareReduce the amount requiredAntibacterial agentsBiocideFreeze-dryingSolvent
An injectable composition comprises a benzimidazole compound having an antiulcer action and a strong alkali (e.g., an alkali metal hydroxide such as sodium hydroxide) in a proportion of about 1 equivalent of the latter relative to 1 mol of the former, and is substantially free from a nonaqueous solvent. The injectable composition may comprise N-methylglucamine, and a saccharide (such as mannitol). The injectable composition may be a freeze-dried preparation. The freeze-dried preparation is dissolvable in or dilutive with a distilled water for injection or an infusion solution without a nonaqueous solvent. The injectable composition is useful as an antiulcer agent.
Owner:TAKEDA PHARMA CO LTD
Power for intravenous injection with liver-protecting action, and its preparation and quality control method
The present invention discloses a powder injection for intravenous injection for curing several diseases of liver damage with obvious therapeutic effect. Said powder injection has the action of protecting liver, its main component is silibinin-N-methylglucamine whose content is 20-500 mg / g.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical formulations of HDAC inhibitors
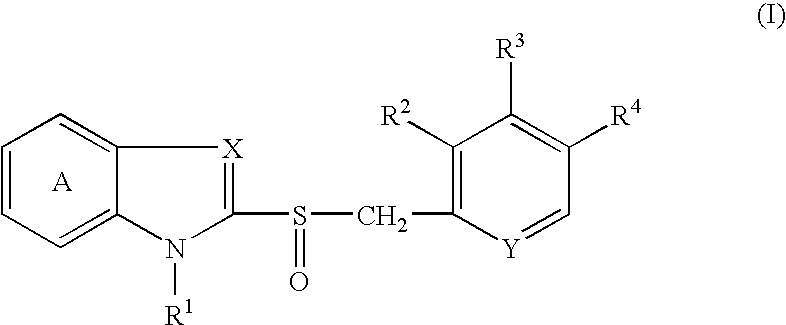
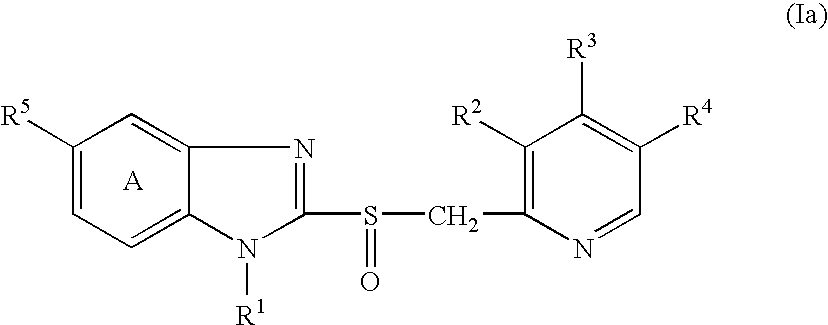
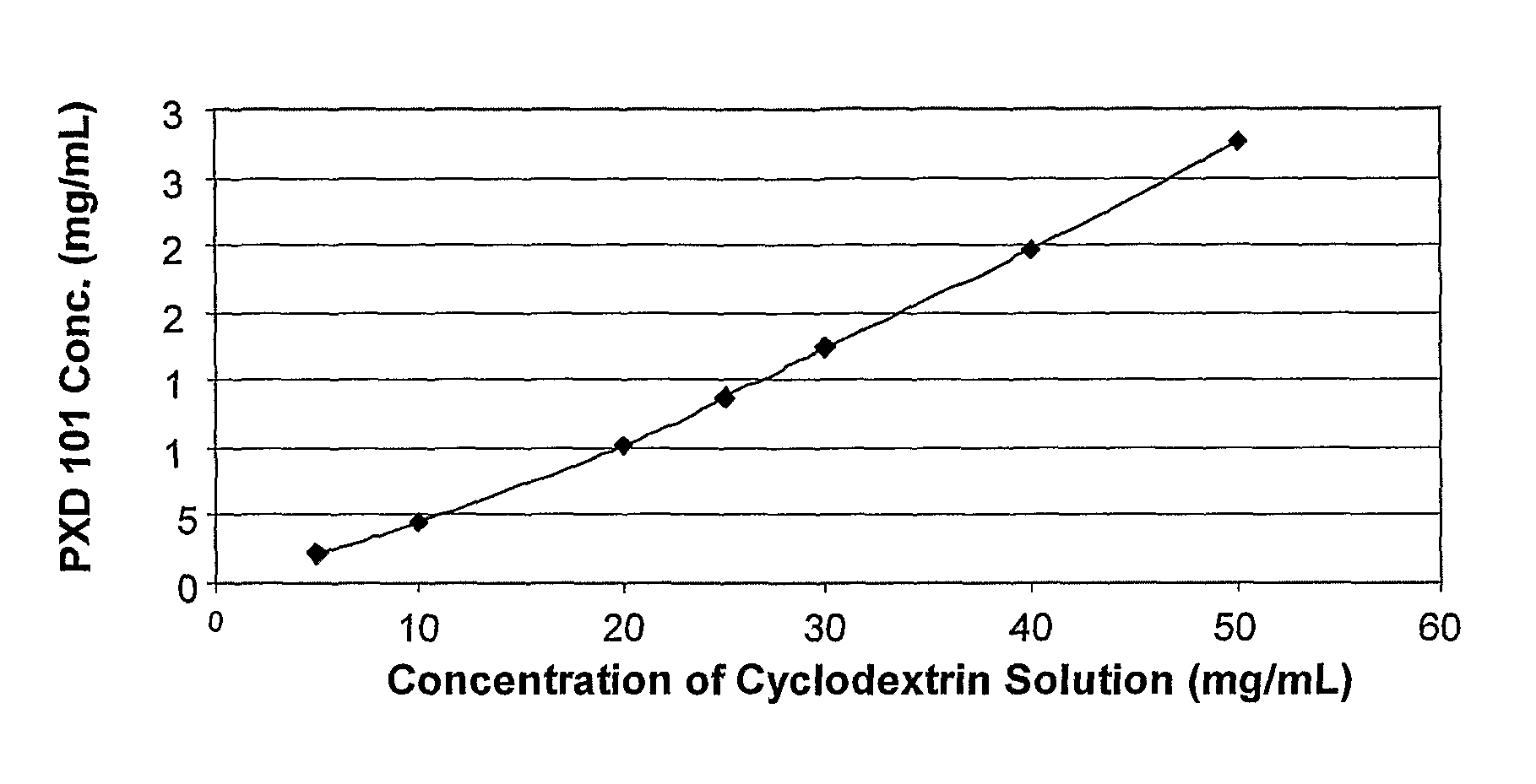
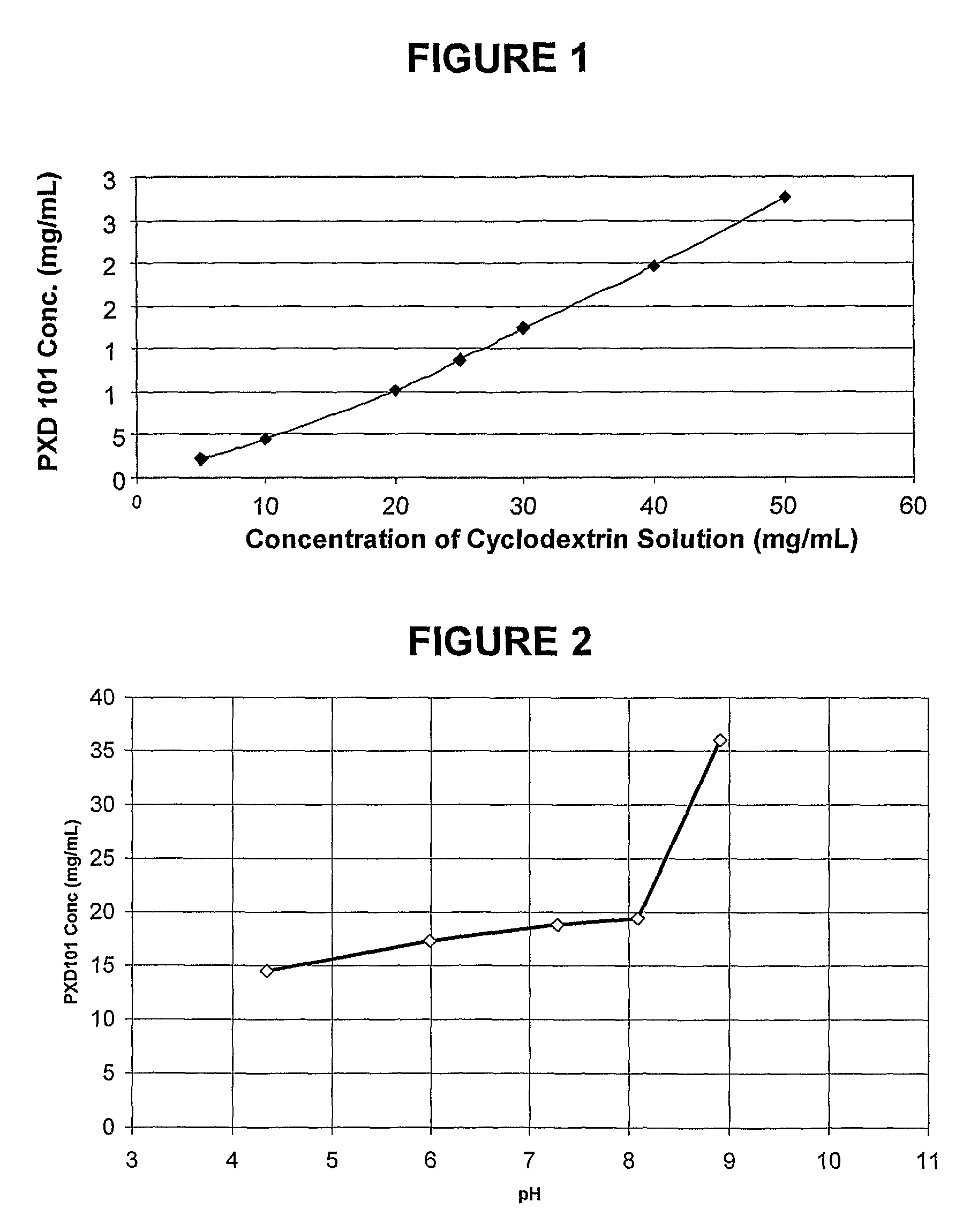
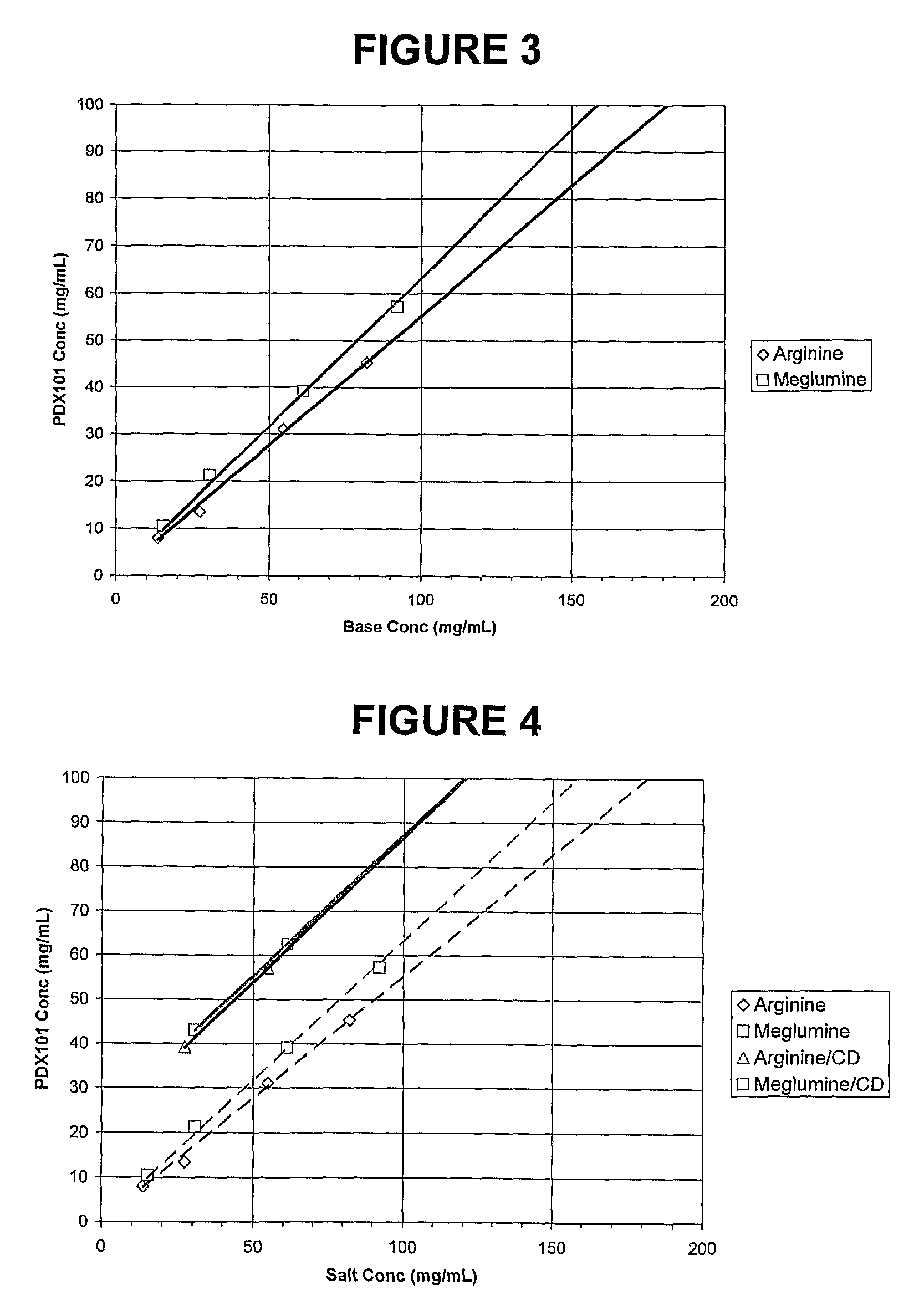
This invention pertains to pharmaceutical compositions comprising certain carbamic acid compounds (e.g., which inhibit HDAC (histone deacetylase) activity) (e.g., PXD-101, N hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide)) and one or more additional ingredients selected from cyclodextrin, arginine, and meglumine. The present invention also pertains to the use of such compositions, for example, in the inhibition of HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Medicinal preparation containing repaglinide and preparation of medicinal preparation
ActiveCN103181923ASimple processSuitable for industrial production needsOrganic active ingredientsMetabolism disorderAdjuvantAlcohol
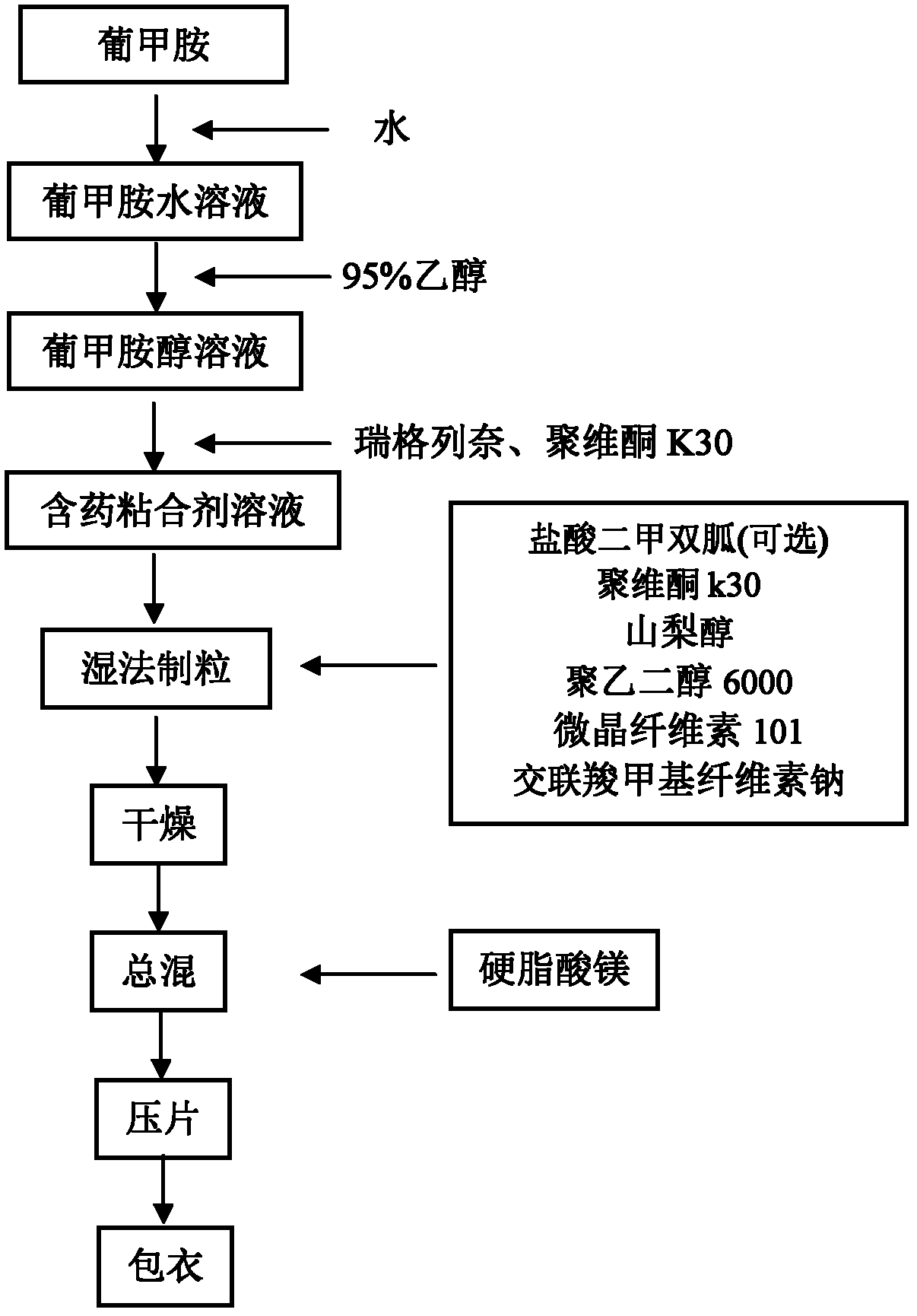
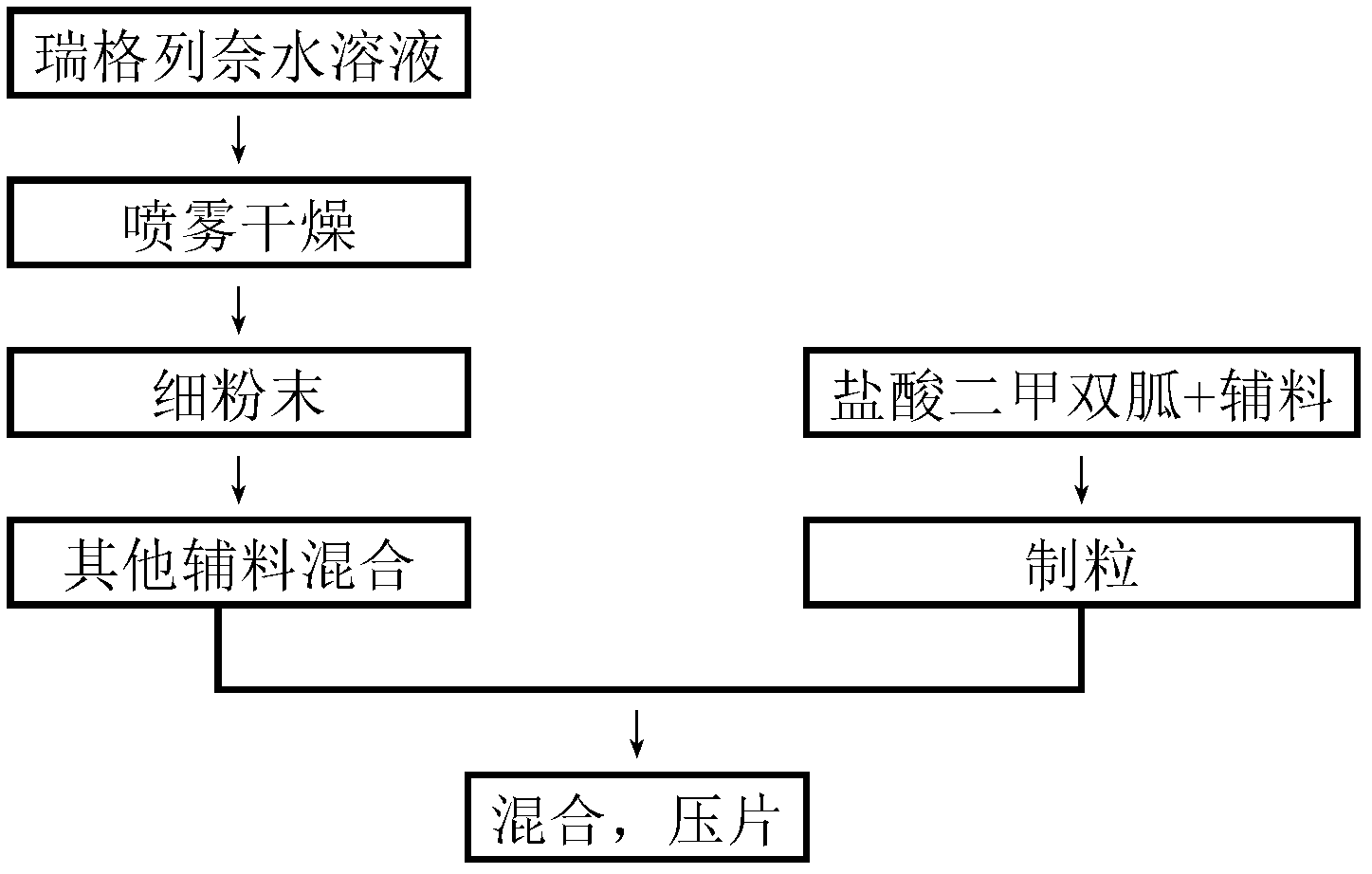
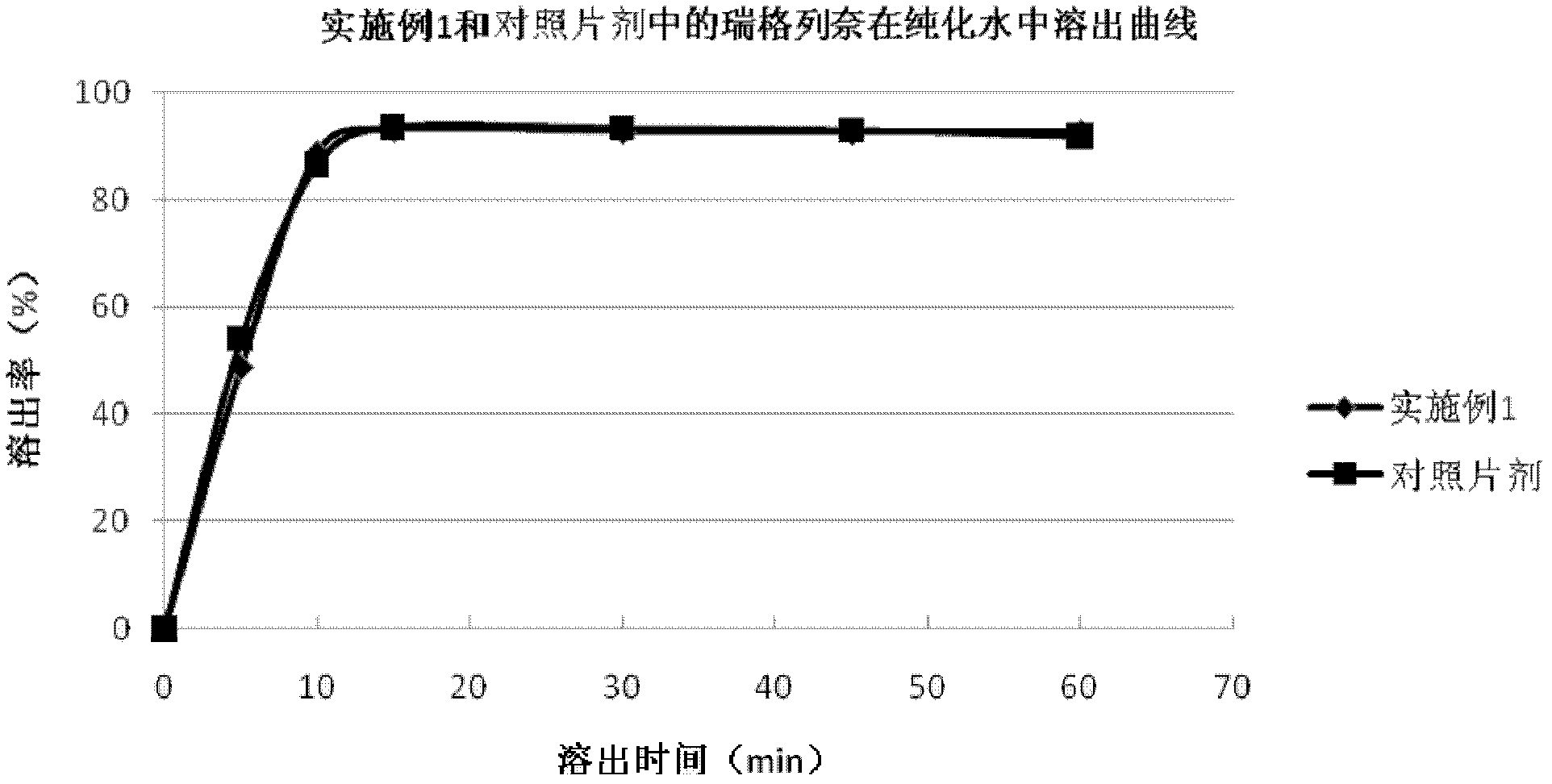
The invention relates to a preparation method of a medicinal preparation of repaglinide. The preparation method comprises the following steps: (a) dissolving meglumine and repaglinide in alcohol and water to prepare an adhesive solution; (b) mixing pharmaceutically acceptable adjuvant materials to prepare a mixture; (c) adding the adhesive solution to the mixture obtained in the step b, and performing wet granulation; and (d) preparing the medicinal preparation. The invention further relates to application of meglumine as a repaglinide stabilizer in the medicinal preparation containing repaglinide.
Owner:BEIJING HANMI PHARMA CO LTD
Novel process for preparation of silybum mariamum extractive methylglucamine
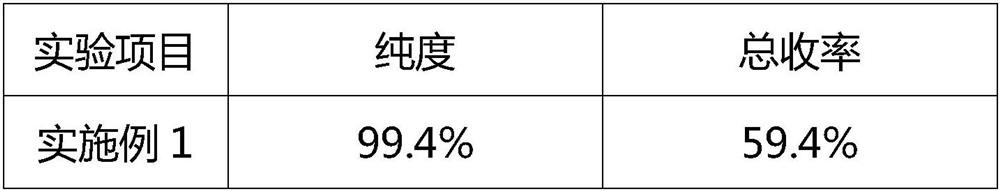
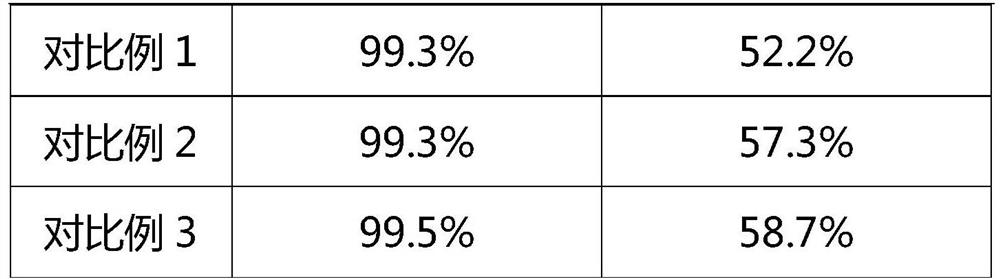
The invention relates to the field of pharmaceutical, in particular a novel process for preparation of silybum mariamum extractive methylglucamine, wherein dissolvent method is employed for preparing silybum mariamum extractive methylglucamine from silybum marianum extract at nonrefluence state, compared with the prior art, this method eliminates heating backflow, dissolvent decompression reclamation and long period of decompression drying, the content of silibinin-N-methylglucamine is over 99.0%.
Owner:NANJING CHENXIANG MEDICAL RES
Rheinic acid derivatives and treatment application thereof
ActiveCN102225913AChange physical and chemical constantsImprove pharmacological activityEsterified saccharide compoundsOrganic active ingredientsDiseaseArginine
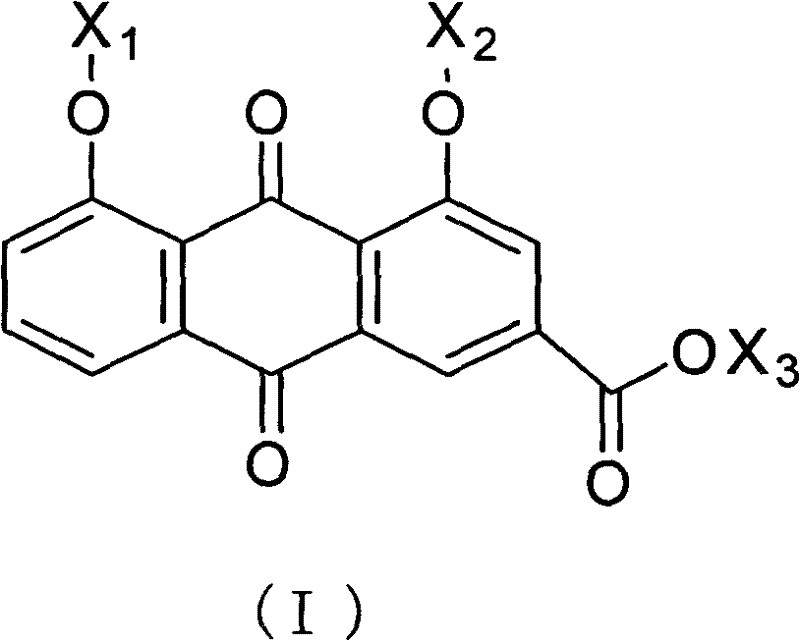
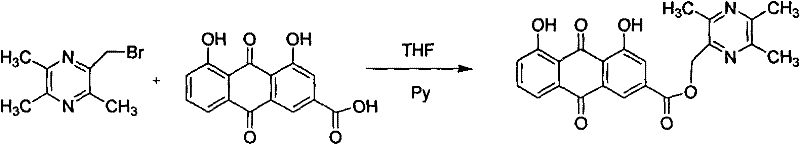
The invention discloses rheinic acid derivatives and treatment application thereof, belonging to the field of medicine. A compound with a bioactivity is a compound as shown in a general formula (I) or salts, solvates or hydrates thereof, wherein X3 is the residue of the compound with the bioactivity, and X1 and X2 are independently H or acetyl individually; X3 is the residue of ligustrazine, arginine, lysine or carnitine; X3 is the residue of meglumine, glucosamine or glucose; and X3 is the residue of meglumine, glucosamine or glucose acetylate. The treatment application is as follows: the rheinic acid derivatives can be used for preparation of medicaments for treating or preventing heart cerebrovascular diseases, and can also be used for preparation of medicaments for treating or preventing diseases related to T-cell proliferation or diseases mediated by pro-inflammatory cytokines.
Owner:南通东湖国际商务服务有限公司
Silicibinin-N-methylglucamine disperser for treating hepatitis, and its prepn. method
InactiveCN100348199CFast absorptionEasy to takeOrganic active ingredientsDigestive systemChronic hepatitisIothalamate Meglumine
Owner:王登之
Prescription of silibinin-n-methylglucamine drop pills and preparation process thereof
InactiveCN1875962AHigh speedPromote absorptionOrganic active ingredientsDigestive systemTreatment effectChronic hepatitis
The invention relates to a medicament for treating acute and chronic hepatitis and process for preparation, wherein the medicament is prepared from silibinin-N-methylglucamine as the main constituent, and right mxtrix through a predetermined processing combinations. Compared with the existing dosage forms, the drop pill preparation has the advantages of short technological process, low cost of production and quick-effectiveness.
Owner:孙民富
Liver protecting prepn containing silybin and total arasaponin and its prepn process and quality control method
The present invention is one kind compound Chinese medicine preparation for protecting liver and treating various hepatopathy and its preparation process and quality control method. The compound preparation consists of water soluble silybin derivative silybin meglumine and total arasaponin as the effective components of notoginseng in the weight ratio of 1 to 0.2-2. It is prepared into injection through dissolving, adding polyglycol or propylene glycol as co-solvent, filtering, ultrafiltering and packing; or into powder for injection through dissolving, adding mannitol or glucose as forming agent, filtering, ultrafiltering and freeze drying. Its contents of silybin and total arasaponin and its fingerprint are determined via HPLC process. The compound preparation of the present invention has curative effect obviously higher than its single components, and has the features of high curative effect and high safety.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Stable lornoxicam solution agent, preparation method and application thereof
InactiveCN101278906AImprove solubilityOrganic active ingredientsAntipyreticSolubilityIntramuscular injection
The invention relates to stable lornoxicam solution and a preparation method and an application thereof; the solution contains the lornoxicam or the pharmaceutically-acceptable salt of the lornoxicam, meglumine, potassium phosphate salt and / or sodium phosphate salt, and has the advantages of good solubility, good stability and good security. The solution of the invention is suitable for parenteral administration such as intramuscular injection, intravenous injection or ophthalmic purpose; the solution which is suitable for the invention is selected from injection preparation, lyophilization formulation or spraying desiccant, preferably the lyophilization formulation.
Owner:南京易亨制药有限公司
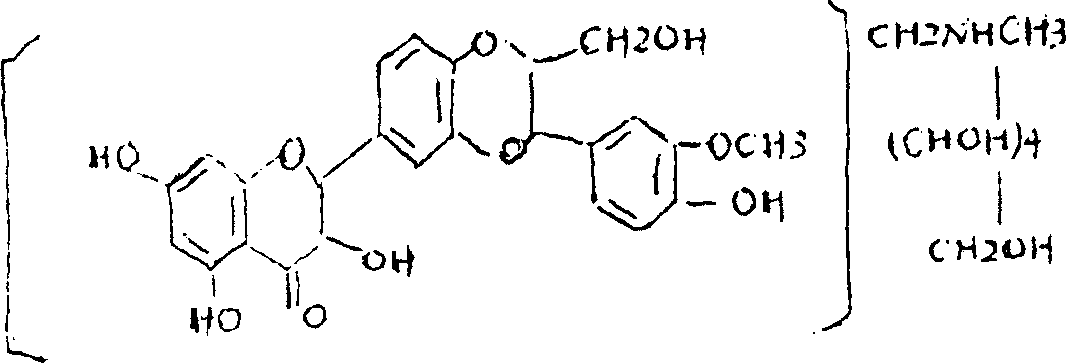
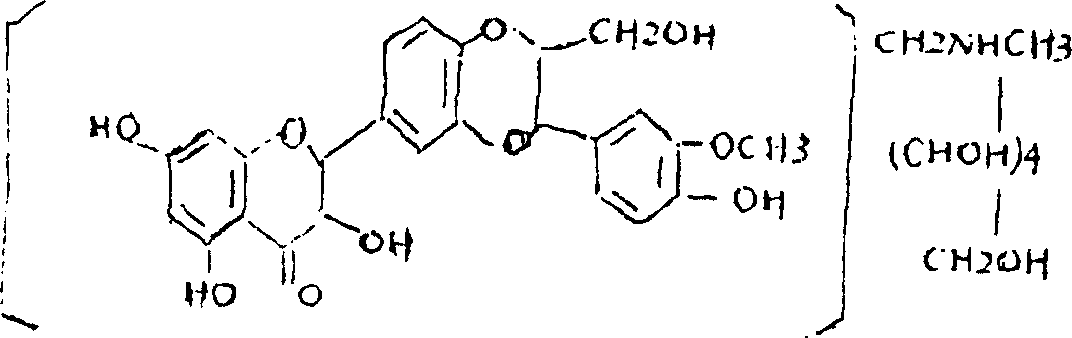
Silymarin Methylglucamine clathrate compound and its preparation method
InactiveCN1565440AImprove bioavailabilityImprove utilizationOrganic active ingredientsDigestive systemMeglumineCombinatorial chemistry
The invention relates to a silymarin meglumine inclusion compound, which is prepared form hepadestal aminomethane and different materials through enclosing technology, the invention also discloses a Silymarin Methylglucamine clathrate compound preparation method.
Owner:烟台同和医药科技有限公司
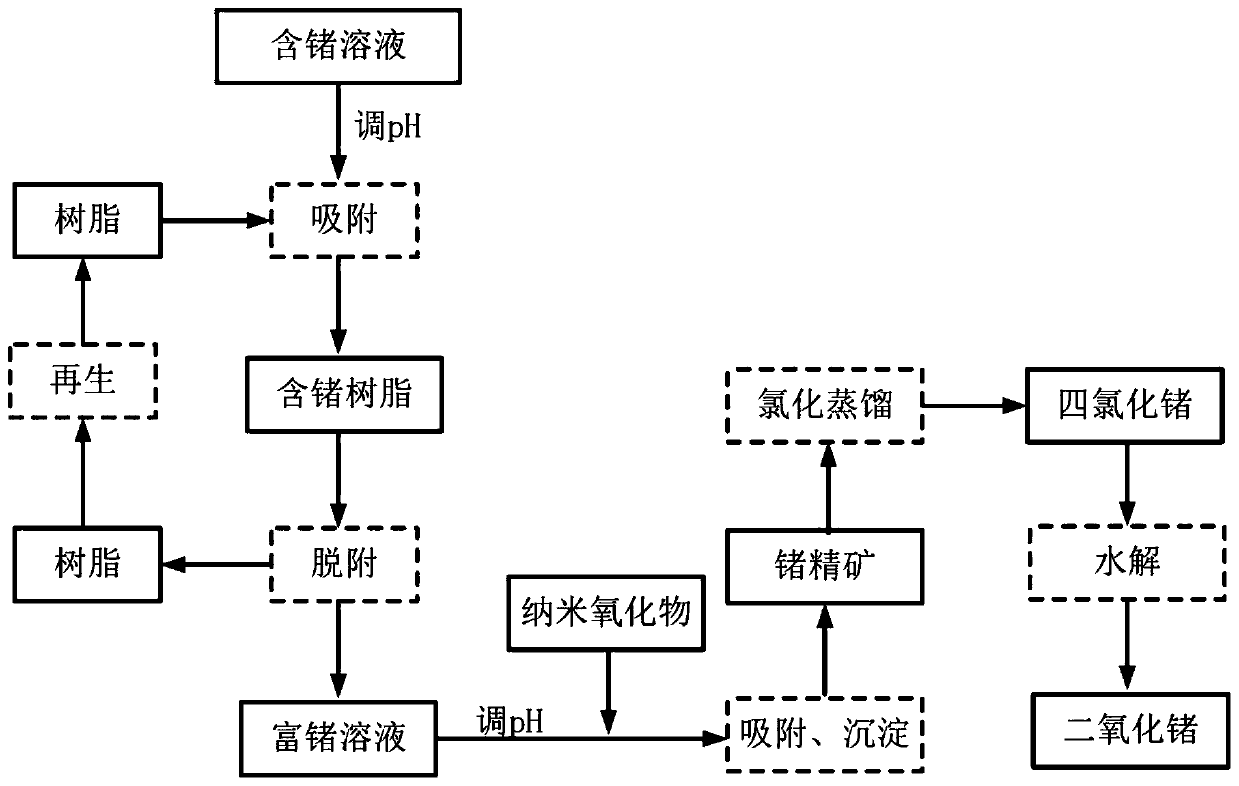
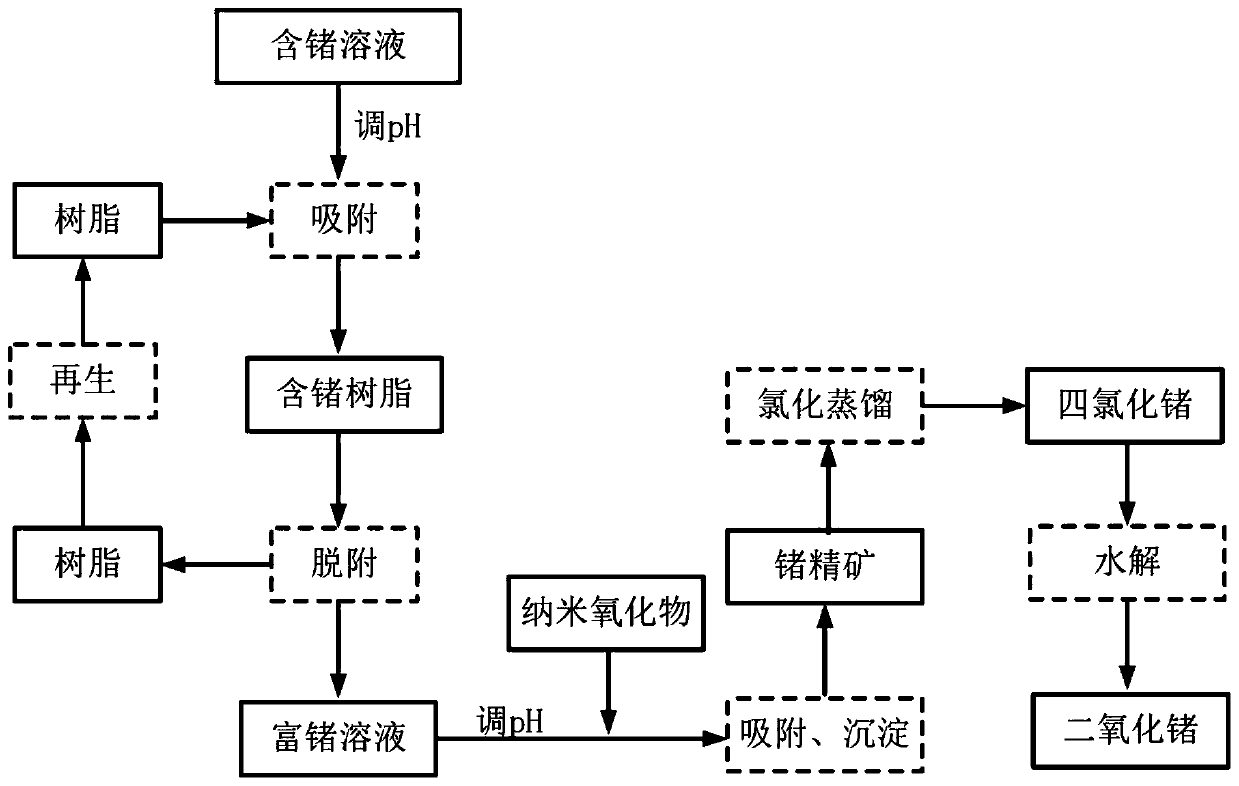
Method for recycling germanium from germanium-containing solution and application of method
ActiveCN111118293AEfficient recyclingImprove the effective recovery rateProcess efficiency improvementAlkaline earth metalDistillation
The invention belongs to the technical field of energy conservation and environmental protection, and particularly relates to a method for recycling germanium from a germanium-containing solution andthe application of the method. The method comprises the following steps that germanium is selectively adsorbed in the germanium-containing solution under an alkaline condition by adopting anion exchange resin containing a meglumine functional group, and germanium desorption is carried out on anion resin loaded with germanium anions under an acidic condition to obtain a primarily enriched germanium-containing acidic solution; the pH value of the germanium-containing acidic solution is adjusted to be alkaline, the germanium in the solution is adsorbed and precipitated by using a nano alkaline-earth metal oxide, and the precipitate is dried to obtain germanium-containing concentrate; and the germanium-containing concentrate is subjected to chlorination distillation to obtain germanium tetrachloride, germanium dioxide is obtained through hydrolysis, and thus germanium recycling is achieved. The method realizes selective adsorption of germanium in the germanium-containing solution, efficient enrichment and low-cost resource recycling can be realized, the problems of high cost, large acid consumption, environmental unfriendliness and the like of traditional tannin germanium precipitation, neutralization precipitation, chlorination distillation and other processes can be effectively solved, and the method has the characteristics of simple process, low cost, environmental friendlinessand the like.
Owner:HUAZHONG UNIV OF SCI & TECH
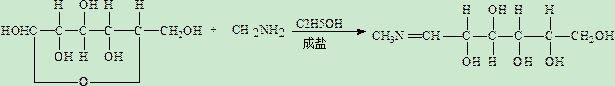
Meglumine production process
ActiveCN112479906AHigh catalytic efficiencyIncrease productivityOrganic compound preparationChemical recyclingEthylene diamine tetra aceticEthylene diamine
The invention relates to a meglumine production process which comprises the following steps: S1, condensing monomethylamine with glucose in absolute ethyl alcohol to obtain a Schiff's salt; s2, addinga catalyst and absolute ethyl alcohol into the Schiff's salt, introducing hydrogen to react to obtain hydride, performing crystallizing to obtain solid hydride, adding water to melt the hydride, adding disodium ethylene diamine tetraacetate, carrying out concentrating under reduced pressure, carrying out centrifugal filtration to obtain a meglumine crude product, and purifying the meglumine crudeproduct, wherein the catalyst is skeleton nickel, and the preparation method of the skeleton nickel comprises the following steps: stirring liquid caustic soda, performing heating to 40-47 DEG C, gradually adding aluminum-nickel-chromium-rhodium alloy powder, naturally performing heating to 85-100 DEG C, keeping the temperature for 2.5-3 hours, performing cooling to 55-65 DEG C, stopping stirring, and performing washing with water and absolute ethyl alcohol. The meglumine production process has the effect of improving the meglumine production efficiency.
Owner:弘健制药(上海)有限公司
Medicine composition containing cefpodoxime proxetil
InactiveCN108261404AImprove stabilityImprove dissolution rateAntibacterial agentsOrganic active ingredientsDissolutionHydrolysis
The invention discloses a medicine composition containing cefpodoxime proxetil. Meglumine is added into the composition to be used as a gelatin inhibitor; the gelation phenomenon of the solid preparation in an aqueous solution can be effectively inhibited; the dissolution-out performance of a solid preparation is effectively improved; the bioavailability is further improved. A flavoring agent of sodium glutamate is added in the composition to achieve the cooperated effect with meglumine, the problem of bitter taste of the particle preparation is solved. Through a dry process granulation process, the introduction of adverse factors such as moisture and high temperature is avoided; a preparation recipe is matched; the problems of hydrolysis or oxidization degradation of ester bonds, amido bonds, lactam bonds and primary amine groups in raw medicine molecules is solved. Experiments prove that the effect is good.
Owner:天津双硕医药科技有限公司
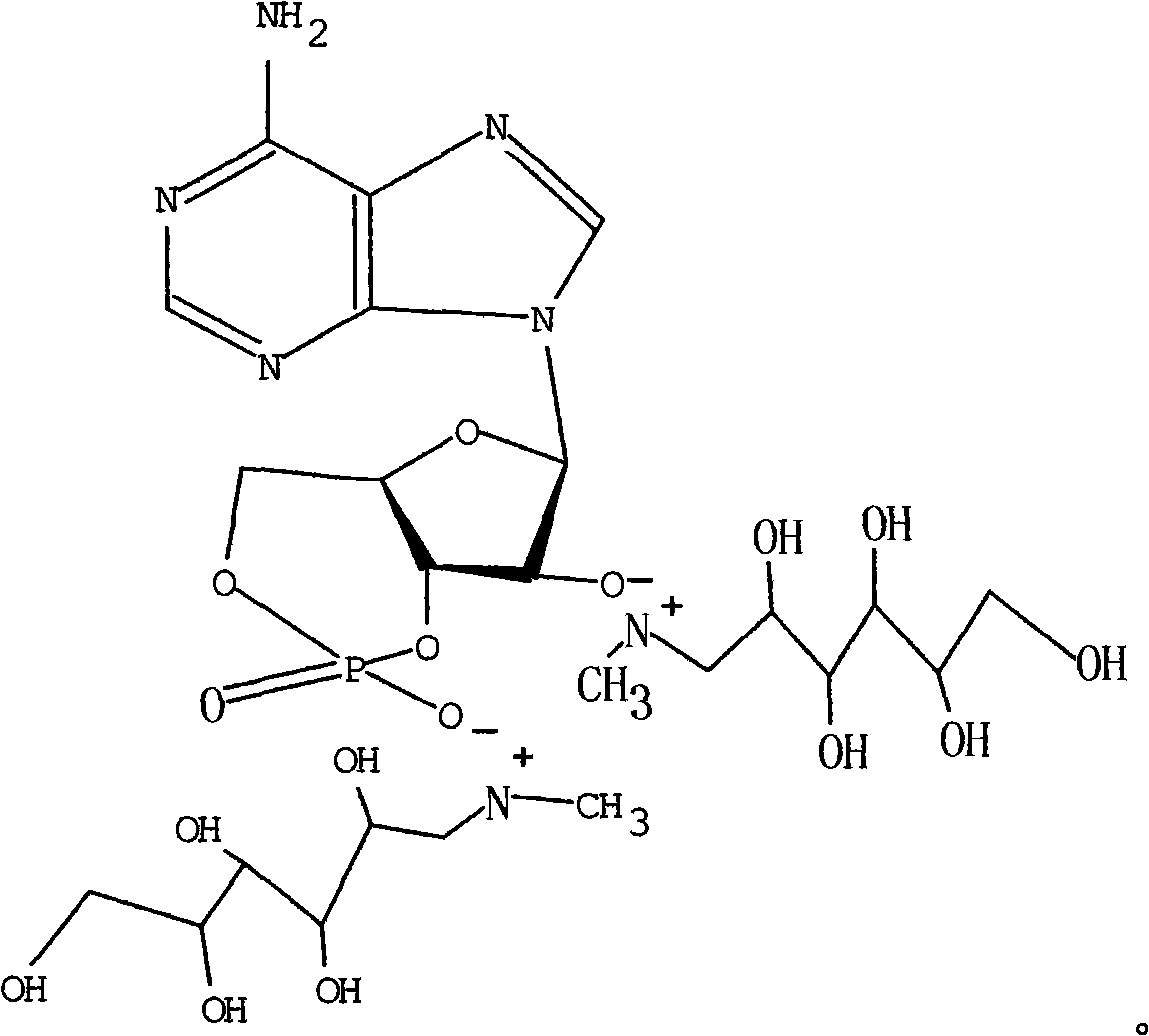
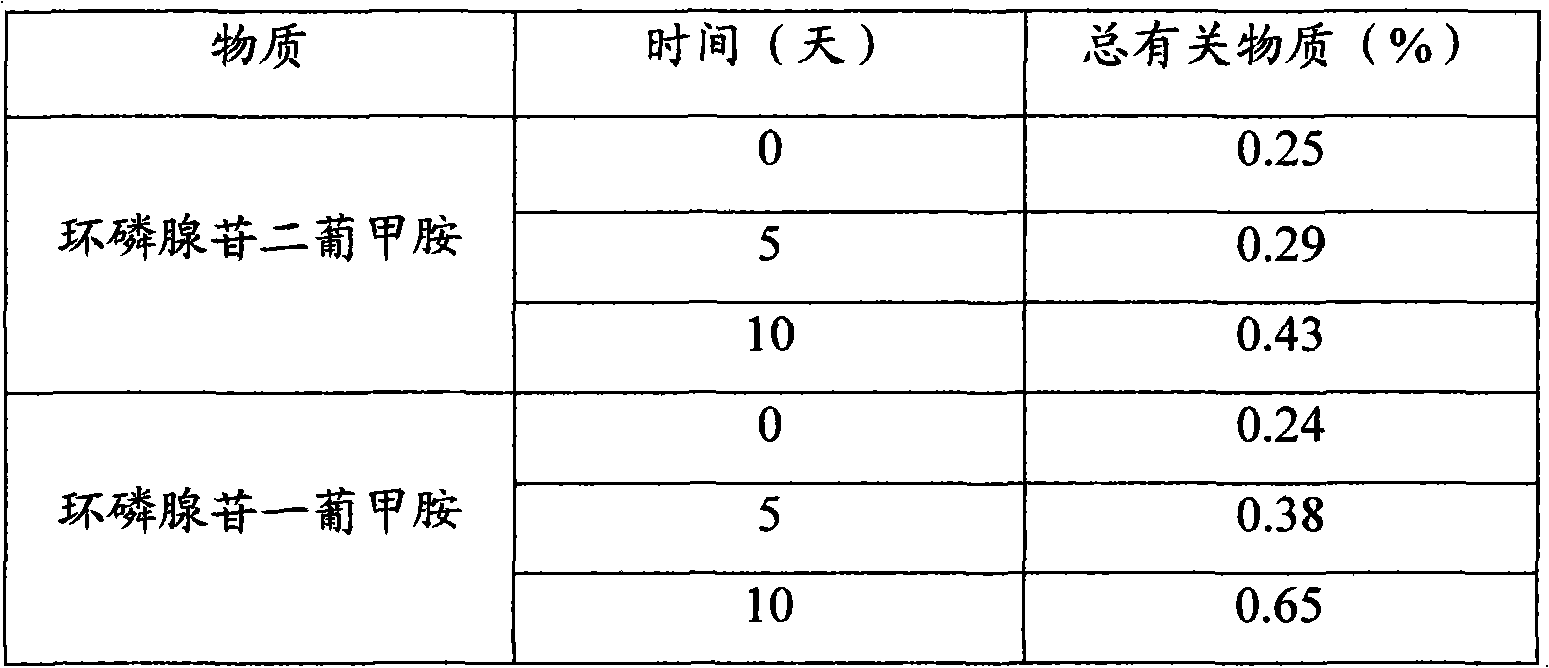
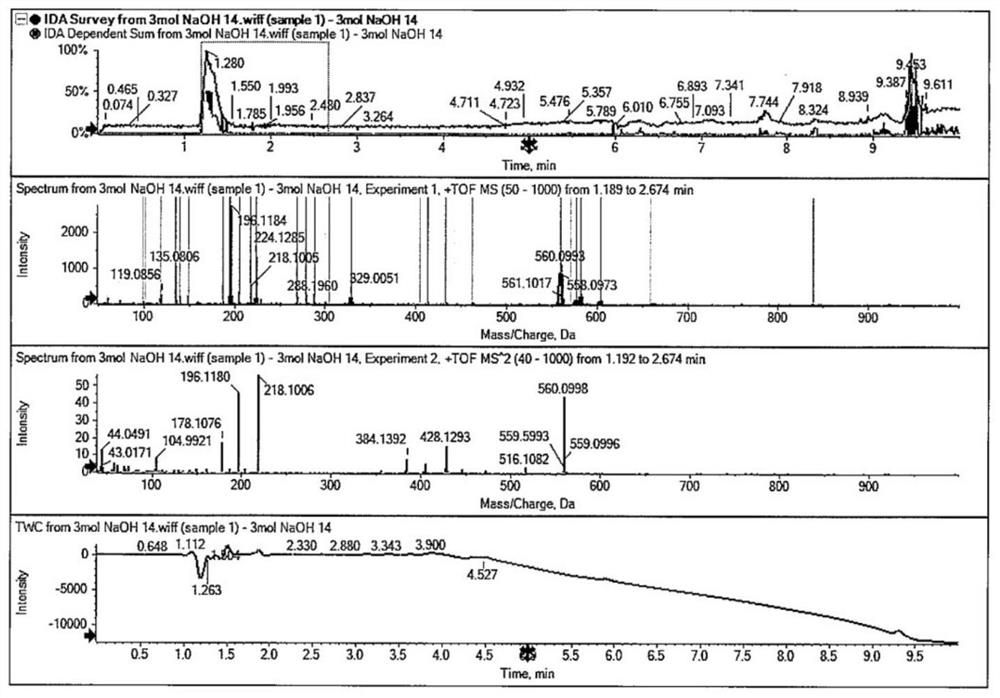
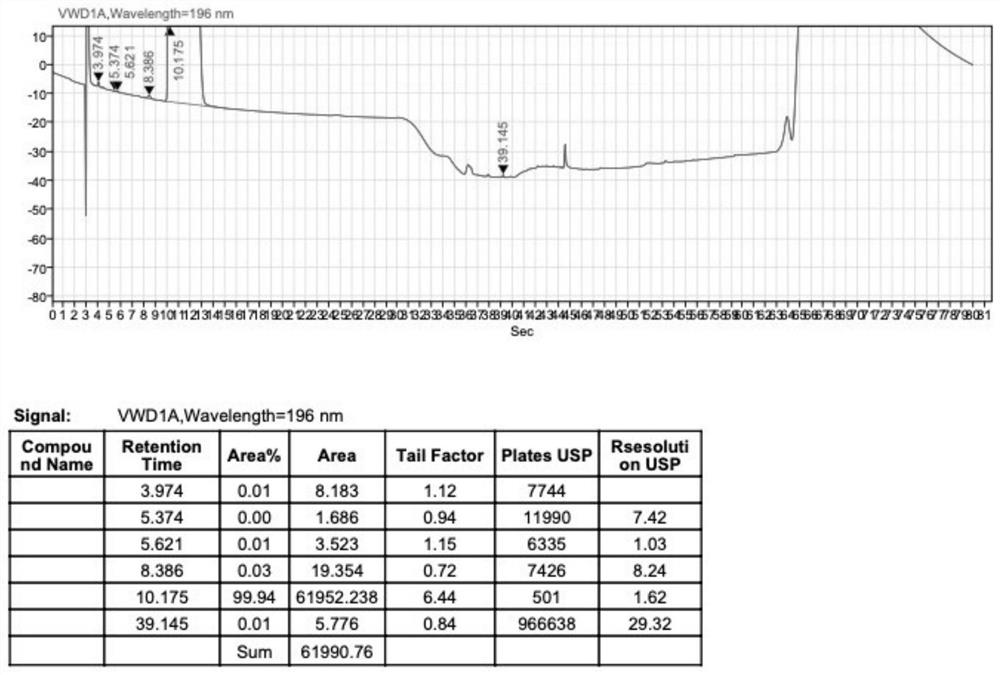
Adenosine cyclophosphate double-molecule meglumine compound and preparation method thereof
ActiveCN102796156AGood water solubilityImprove stabilityOrganic active ingredientsSugar derivativesSolubilityAdenosine
The invention relates to an adenosine cyclophosphate double-molecule meglumine compound and a preparation method thereof, and provides a compound formed by 1 molecule of adenosine cyclophosphate and 2 molecules of meglumine, a preparation method thereof and a medicinal composition comprising the denosine cyclophosphate double-molecule meglumine compound. The water solubility and the stability of the compound are more excellent than those of the conventional compounds.
Owner:宁辉
Lymphocyte separating medium and preparation method thereof
PendingCN113502264AAchieve separationImprove efficiencyCell dissociation methodsBlood/immune system cellsFicollSucrose
The invention discloses a lymphocyte separating medium and a preparation method thereof, the lymphocyte separating medium is characterized by comprising the following components: glycerin, ethanol, sucrose, meglumine, diatrizoic acid and epichlorohydrin; the sucrose and the diatrizoic acid are added into an ethanol solution to synthesize a water-soluble polysucrose ethanol solution as a molecular sieve separating medium; the method also includes adding meglumine and epichlorohydrin into glycerol, preparing a clear biological solution at room temperature, putting the biological solution into a dialysis paper bag, putting the dialysis paper bag into deionized water, carrying out ion replacement culture for a period of time, and detecting that the dialysis paper bag does not contain redundant foreign ions; then adjusting the pH value of the two solutions to a proper range by using sodium hydroxide and hydrochloric acid; finally, mixing and stirring the two solutions with the pH value adjusted to be stable to be uniform, and obtaining the lymph separation medium. The lymphocyte separation efficiency is high, the separation purity is high, the quality of lymphocytes is improved, high-quality lymphocytes can be obtained, and the lymphocyte separation medium is suitable for clinical application and popularization.
Owner:无锡华精生物科技有限公司
Meglumine production process capable of recycling solvent
InactiveCN112608242AReduce contentReduce recycling costsOrganic compound preparationHydroxy compound separation/purificationEthylene diamine tetra aceticEthylene diamine
The invention relates to a meglumine production process capable of recycling a solvent. The meglumine production process comprises the following steps: S1, condensing monomethylamine with glucose in absolute ethyl alcohol to obtain a Schiff's salt; S2, adding a catalyst and absolute ethyl alcohol into the Schiff's salt, introducing hydrogen for a reaction to obtain hydride, conducting crystallizing to obtain solid hydride and mother liquor, separating the solid hydride, melting the hydride, adding disodium ethylene diamine tetraacetate, conducting concentrating under reduced pressure, and carrying out centrifugal filtration to obtain a crude meglumine product; S3, recrystallizing the crude meglumine product in methanol to obtain a methanol mother solution and a wet meglumine product, and distilling the methanol mother solution under reduced pressure to obtain a methanol recovery solution; and S4, adding sulfuric acid into the mother solution obtained in the step S2 to adjust a pH value to 6-7, adding silica gel particles, continuously conducting stirring and uniform mixing, filtering out the silica gel particles to obtain a filtrate, and conducting distilling to obtain an ethanol recovery solution. The process of the invention has the effect of improving ethanol recovery and dehydration effect.
Owner:弘健制药(上海)有限公司
Refining method of gadadotec acid meglumine
ActiveCN113801071AOrganic compound preparationAmino-hyroxy compound preparationCalcium silicateGadolinium oxide
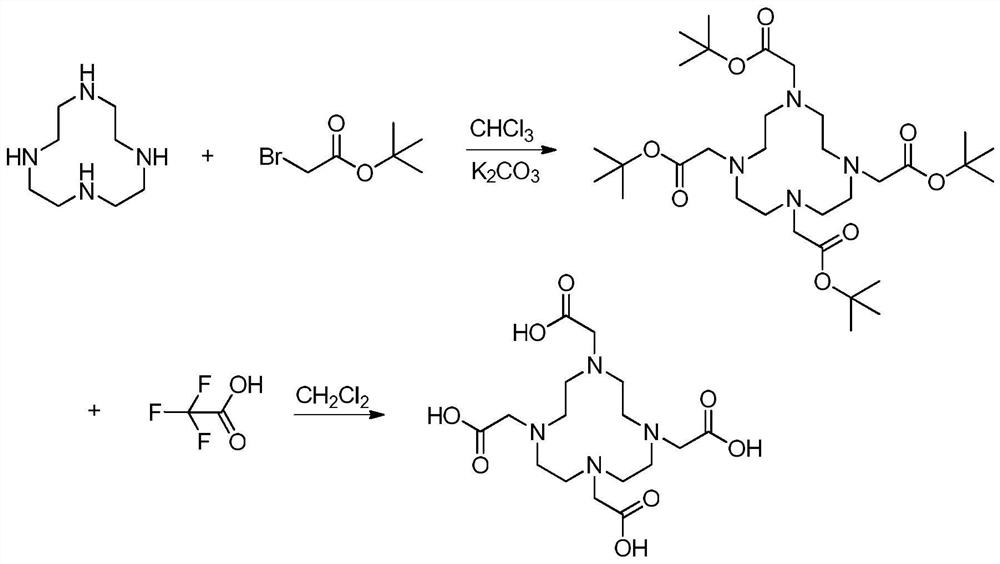
The invention provides a preparation process and a purification process of gadadotec acid meglumine. The preparation process comprises the steps of adding purified DOTA into pure water, stirring and dissolving at room temperature, adding gadolinium oxide and microporous calcium silicate particles, adding meglumine until the pH value of the solution is 7-9, carrying out ultrasonic oscillation, filtering, heating the filtrate to the temperature of 40-50 DEG C, stirring, reacting for 2-4 hours, filtering the precipitate, and performing vacuum drying to obtain the product. The purification method provided by the invention comprises the steps of dissolving a crude product gadadotec acid meglumine in 100ml of methanol, heating while stirring until the gadadotec acid meglumine is dissolved, adding 50ml of water while stirring, stopping stirring, naturally cooling, slowly separating out fine crystals, cooling to the temperature of 5-10 DEG C after 3 hours, adding 30ml of water while stirring, stopping stirring after adding, standing for crystallization, and carrying out suction filtration on the separated white crystals, washing with a small amount of methanol, and carrying out vacuum drying at the temperature of 60 DEG C to obtain crystalline powder.
Owner:ANHUI POLY PHARM CO LTD +1
Water-soluble aureomycin succinic acid monoester salt and preparation method thereof
ActiveCN105001112BGrowth-promoting effectImprove immunityAntibacterial agentsOrganic compound preparationChemical synthesisArginine
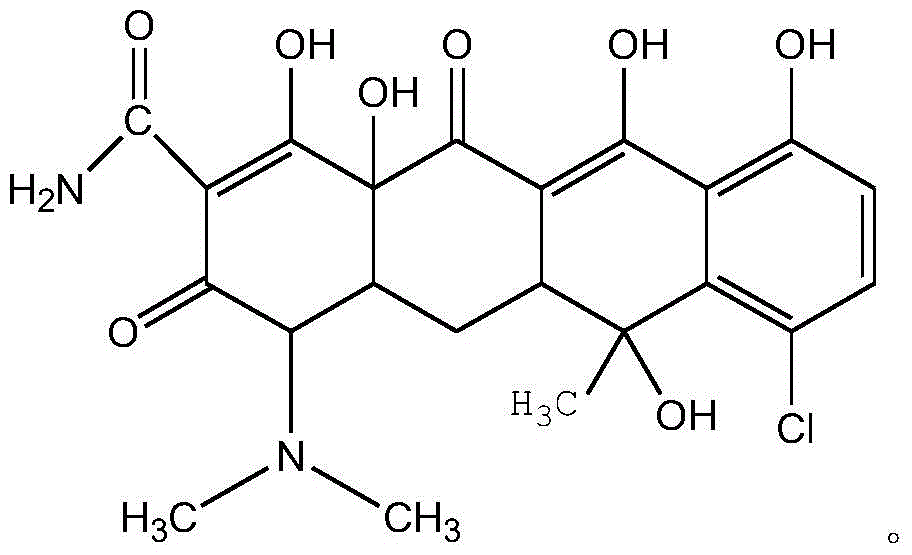
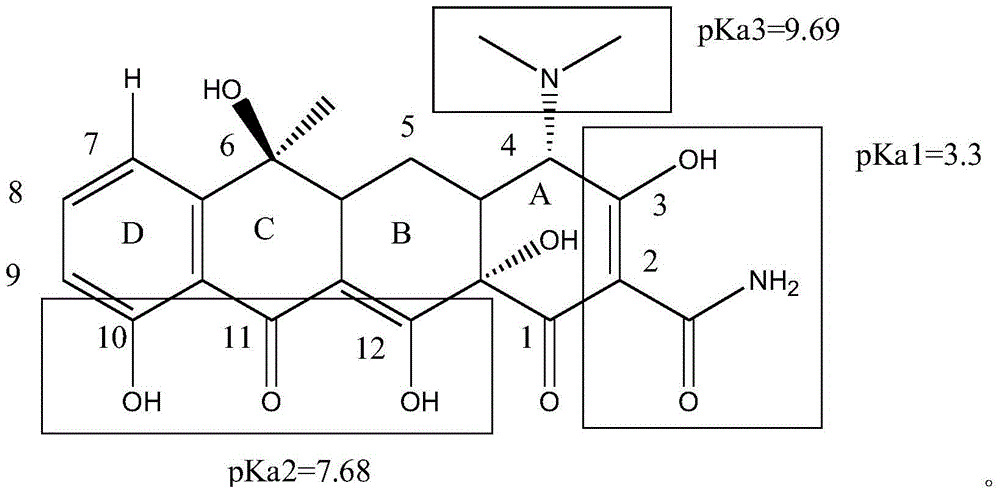
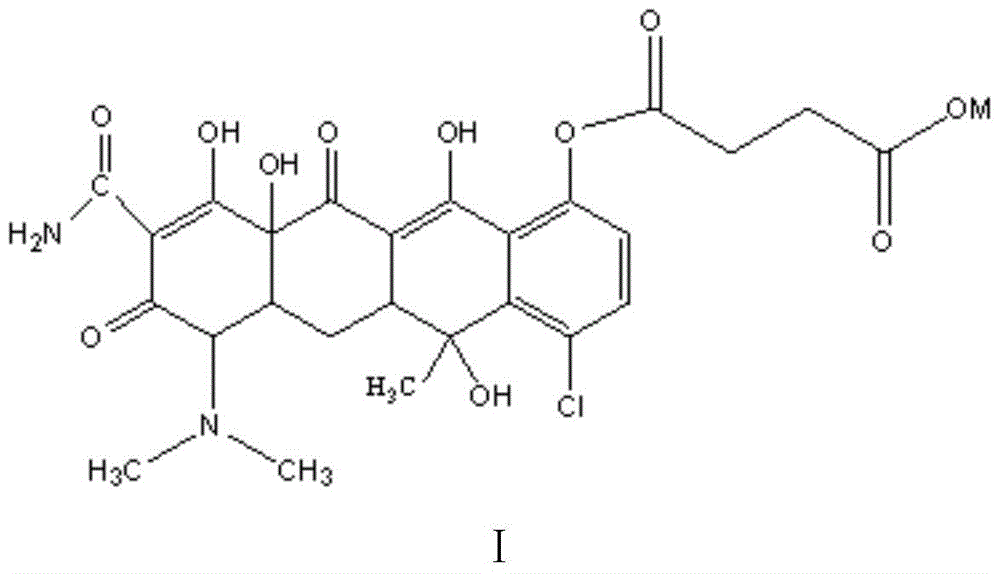
The invention belongs to the technical field of chemical synthesis, and more specifically relates to a water soluble chlorotetracycline succinic acid monoester salt, and a preparation method thereof. The preparation method is used for modifying chlorotetracycline. The chlorotetracycline succinic acid monoester salt possesses a structure represented by formula I, wherein M is used for representing Na, K, meglumine, arginine, or lysine. According to the preparation method, grafting modification of chlorotetracycline with succinic anhydride is carried out so as to obtain the chlorotetracycline succinic acid monoester salt with water solubility better than that of chlorotetracycline, and broad-spectrum antibacterial property of the water soluble chlorotetracycline succinic acid monoester salt is the same as that of chlorotetracycline in animal bodies.
Owner:PUCHENG CHIA TAI BIOCHEM
Power for intravenous injection with liver-protecting action, and its preparation and quality control method
The present invention discloses a powder injection for intravenous injection for curing several diseases of liver damage with obvious therapeutic effect. Said powder injection has the action of protecting liver, its main component is silibinin-N-methylglucamine whose content is 20-500 mg / g.
Owner:LUNAN PHARMA GROUP CORPORATION
Nrf2 activators
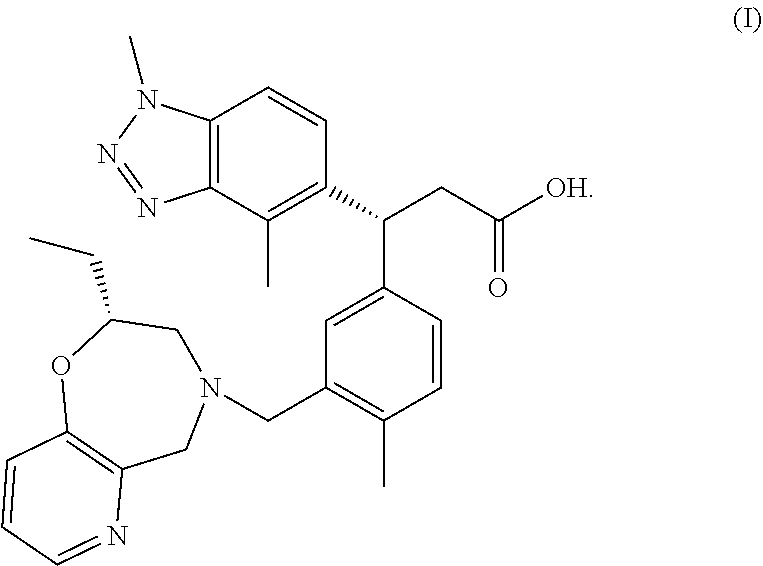
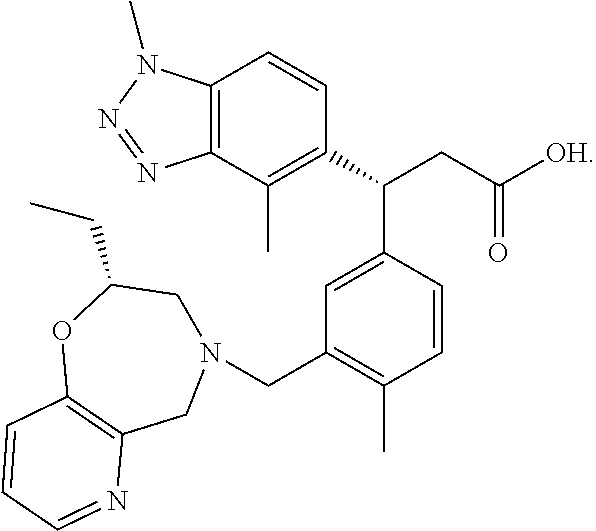
The present invention relates to a compound which is (R)-3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-(3-(((R)-2-ethyl-2,3-dihydropyrido[2,3-f][1,4]oxazepin-4(5H)-yl)methyl)-4-methylphenyl)propanoic acid (I), or a pharmaceutically acceptable salt thereof, in particular, the meglumine salt thereof, a pharmaceutical composition containing the compound and its use as an NRF2 activator.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
A kind of crocetin solid dispersion and preparation method thereof
ActiveCN112999162BImprove the cumulative dissolution rate in vitroImprove bioavailabilityOrganic active ingredientsPowder deliverySolvent evaporationPharmaceutical drug
The invention provides a crocetin solid dispersion and a preparation method thereof. The formula of the crocetin solid dispersion is as follows: 1 part of insoluble drug crocetin, 1-15 parts of a hydrophilic polymer carrier material, 1-12 parts of solubilizing agent meglumine; the preparation method of the described solid dispersion, the steps are as follows: take the hydrophilic polymer carrier material and meglumine, dissolve them in organic reagents or water, ultrasonically or stir to make the hydrophilic The polymer material and meglumine are cross-linked to form a carrier polymer I, adding crocetin acid to the above solution containing carrier polymer I, ultrasonically or stirring to dissolve the crocetin acid, and using solvent evaporation or spray drying method to prepare solid dispersions. The crocetin solid dispersion prepared by the invention has good solubility and stability, rapid absorption in the body, high bioavailability, simple preparation process and is suitable for industrial production.
Owner:HANGZHOU JIURUTANG BIOTECH
Polyionic infusion solution
ActiveCN111372573AOrganic active ingredientsInorganic non-active ingredientsButanedioic acidPharmaceutical industry
The invention relates to the pharmaceutical industry and to medicine, and more particularly to complex polyionic infusion solutions that have a detoxifying effect as a result of antihypoxic, antioxidant and hepatoprotective properties. A preparation can be used to treat intoxications of various origins. The present polyionic infusion solution comprises chlorides of sodium, potassium and magnesium,meglumine sodium succinate as a biologically active component, and water for injection, and also a stabilising agent which is a pharmaceutically acceptable carboxylic acid or inorganic acid or a combination thereof, wherein the solution has a pH from 7.0 to 5.5.
Owner:玻利萨恩科技制药有限责任公司
Compound preparation for reducing hepatotoxicity of tripterygium glycoside tablets, and preparation method of compound preparation
InactiveCN111297814AReduce liver toxicityGood curative effectOrganic active ingredientsAntipyreticEfficacyBiology
The invention discloses a compound preparation for reducing hepatotoxicity of tripterygium glycoside tablets, and a preparation method of the compound preparation. The compound preparation comprises silibin meglumine tablets and the tripterygium glycoside tablets. The compound preparation has the efficacies of protecting the liver for detoxification, tonifying the kidney and strengthening the spleen; the dosage of tripterygium glycosides can be obviously increased; a better curative effect is achieved; and meanwhile, the hepatotoxicity of the tripterygium glycosides is obviously reduced. In addition, according to the compound preparation, the fast release of silibin meglumine and the long-acting stable release of tripterygium glycosides can be realized, and the treatment effect is furtherimproved. In addition, the preparation method provided by the invention has the advantages that the operation is simple; the cost is low; the preparation method is suitable for industrial production;and the application prospects are good.
Owner:HUNAN QIANJIN XIELI PHARMA CO LTD
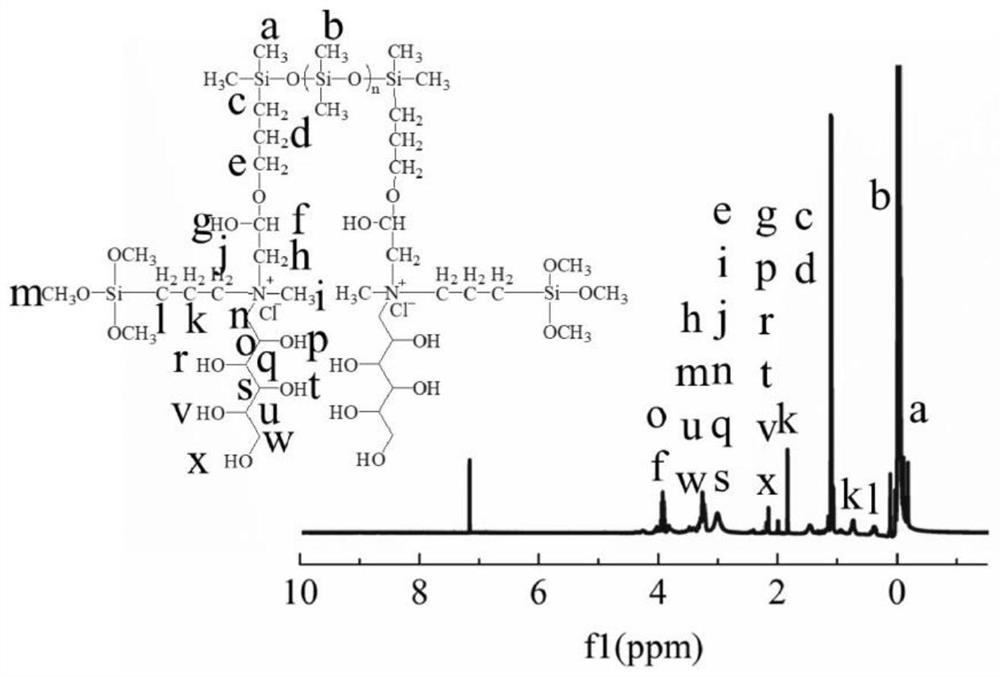
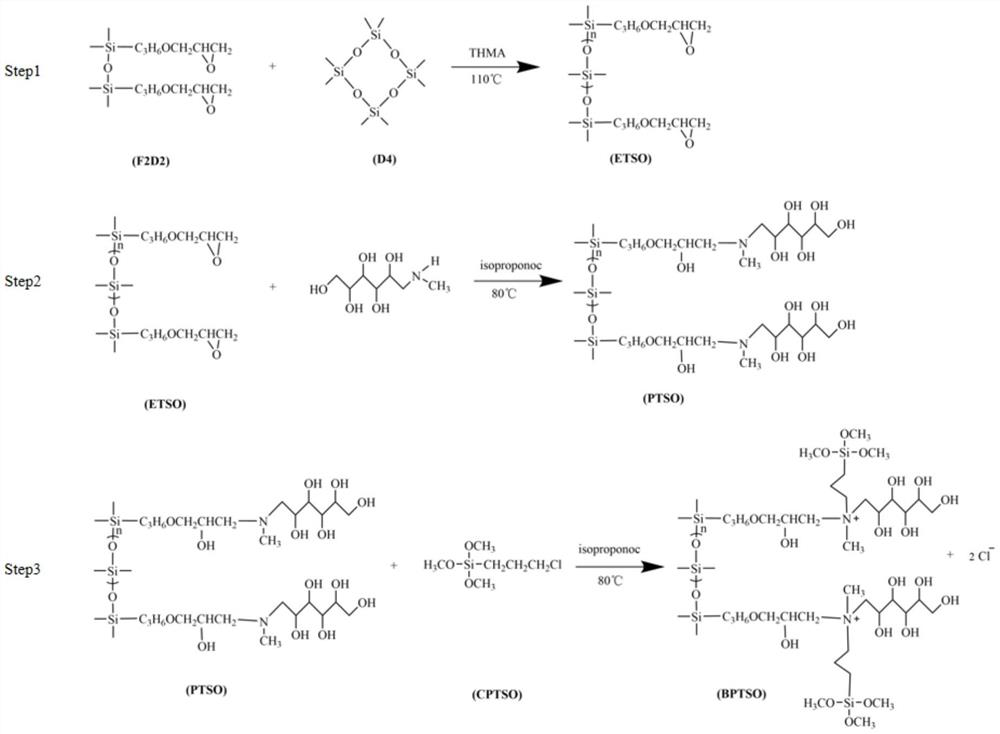
A kind of bola type organosilicon quaternary ammonium salt and its preparation method and application
ActiveCN112409598BHigh whitenessGood flexibilityBiochemical fibre treatmentGrip property fibresEpoxyPolymer science
The invention provides a preparation method of Bola type organosilicon quaternary ammonium salt, comprising the following steps: A) under protective atmosphere conditions, carrying out addition reaction of allyl glycidyl ether and tetramethyldisiloxane to obtain Epoxy double head F 2 D. 2 ; B) the epoxy double head F 2 D. 2 Mixing and heating with octamethylcyclotetrasiloxane for chain extension reaction to obtain epoxy-terminated silicone oil ETSO; C) mixing and heating the epoxy-terminated silicone oil ETSO and meglumine for amination reaction, Obtaining the organosilicon block silicone oil PTSO; D) mixing and heating the organosilicon block silicone oil PTSO and a quaternizing agent to perform a quaternization reaction to obtain a Bola-type organosilicon quaternary ammonium salt. The Bola type organosilicon quaternary ammonium salt provided by the invention has obvious whiteness, and can simultaneously improve the whiteness, softness, hydrophilicity and antibacterial properties of the treated fabric.
Owner:GUANGDONG UNIV OF TECH
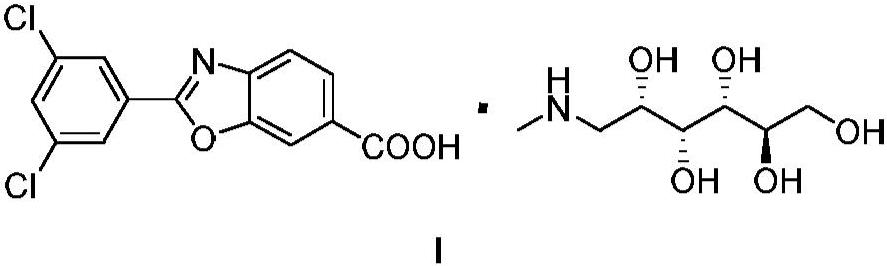
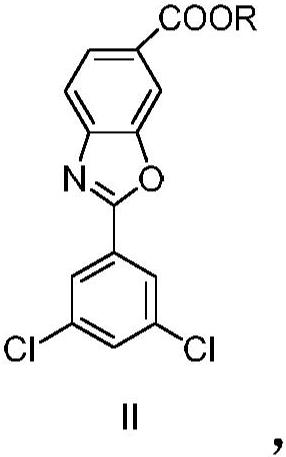
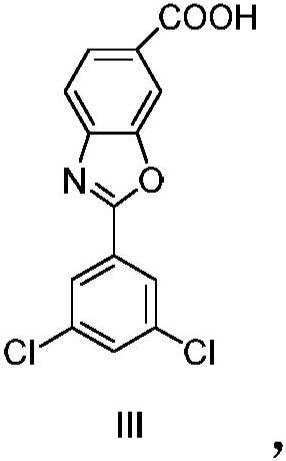
Process for preparing 1-deoxy-1-methylamino-d-glucitol 2-(3,5-dichlorophenyl)-6-benzoxazolecarboxylate
The present invention relates to an improved process for the preparation of 1-deoxy-1-methylamino-D- glucitol 2-(3,5-dichlorophenyl)-6-benzoxazolecarboxylate, also known as tafamidis meglumine. The process of the present invention is particularly suitable for industrial scale manufacture of tafamidis meglumine with excellent purity and high yields.
Owner:INKE股份公司
Preparation method of silymarin skin care product
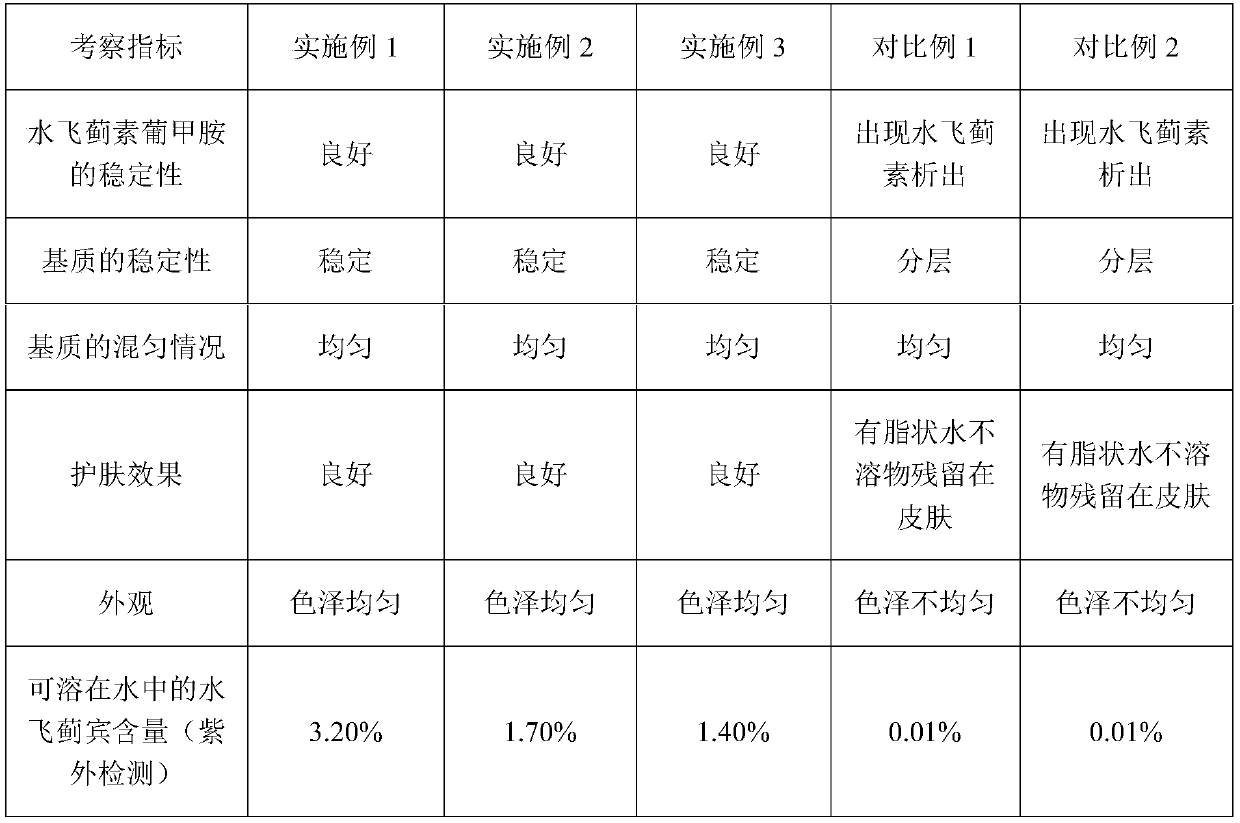
ActiveCN110772447ATroubleshoot unstable technical issuesAvoid destructionCosmetic preparationsToilet preparationsFacial skinAlcohol
The invention discloses a preparation method of a silymarin skin care product. The preparation method comprises the following steps: dissolving the silymarin and meglumine in absolute ethyl alcohol, heating the mixture and then recovering the absolute ethyl alcohol, thereby obtaining a silymarin-meglumine raw material; and evenly mixing the silymarin-meglumine raw material with deionized water, placing the mixture in a stirrer, stirring the mixture, adding gelatin and glycerol, further stirring the mixture, adding sodium benzoate and carrying out an ultrasonic processing, thereby obtaining thedesired silymarin skin care product. The invention solves the defects that the traditional preparation method directly adopts the silymarin to prepare related skin care products, resulting in excessive facial skin grease and pore blockage after long-term use; and the silymarin-meglumine and the sodium benzoate are uniformly distributed in other matrixes by ultrasonic even-mixing technology, and the silymarin-meglumine can not be decomposed or damaged.
Owner:ZHONGXING PHARM CO LTD JIANGSU
Synthesis method of fosaprepitant dimeglumine
ActiveCN111662329AAvoid inactivationHigh activityGroup 5/15 element organic compoundsPalladium on carbonPtru catalyst
The invention discloses a synthesis method of fosaprepitant dimeglumine, and belongs to the technical field of medicines. The synthesis method of fosaprepitant dimeglumine mainly comprises the following steps: reacting tetrabenzyl pyrophosphate, adsorbing impurity tar, hydrogenating palladium mesoporous carbon, refining and the like. According to the invention, a one-pot reaction is adopted; a dibenzyl ester product is generated; sodium carbonate is added for quenching reaction, mesoporous carbon is added for adsorbing impurities and tar, palladium-carbon catalyst deactivation is avoided, thena tetrahydrofuran system is directly utilized for a reaction, palladium mesoporous carbon has high activity and can generate monobenzyl ester first and then remove groups through hydrogenation compared with a common route, steps are saved, and high yield is achieved.
Owner:LIANYUNGANG GUIKE PHARMA
Magnetic resonance contrast agent as well as preparation method and application thereof
ActiveCN113372384AGood water solubilityHigh relaxation efficiencyGroup 5/15 element organic compoundsIron group organic compounds without C-metal linkagesNMR - Nuclear magnetic resonancePharmaceutical medicine
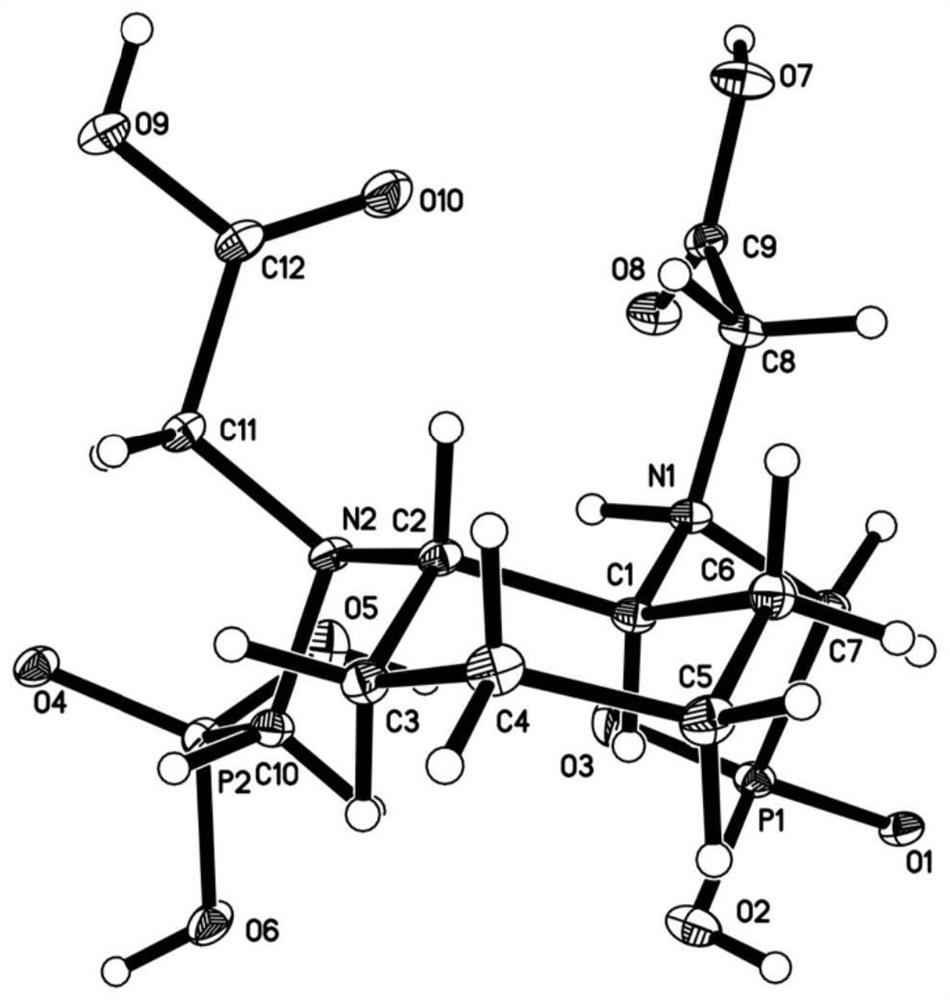
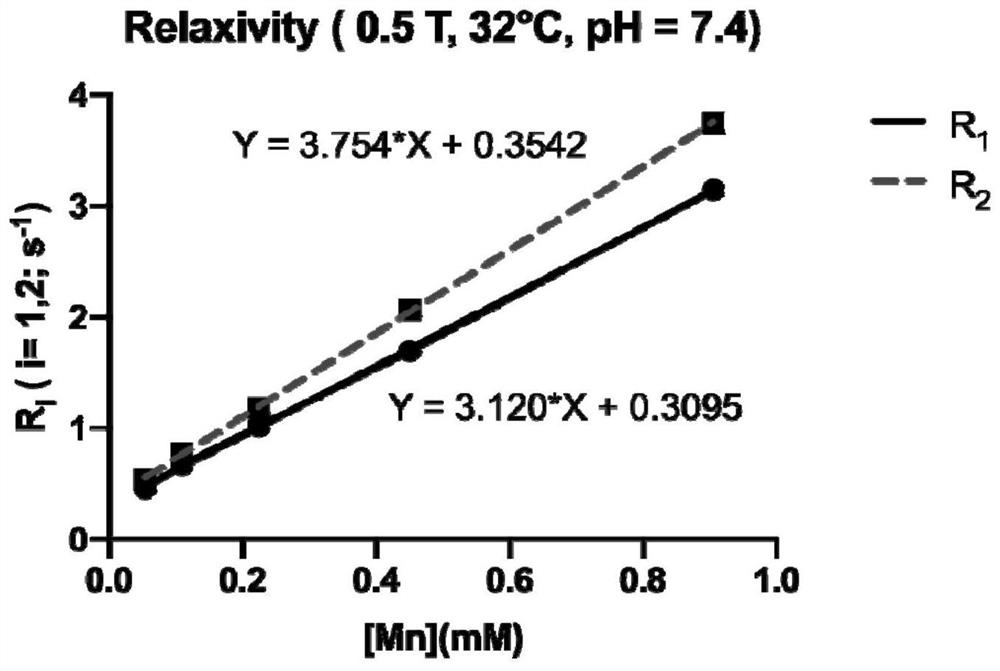
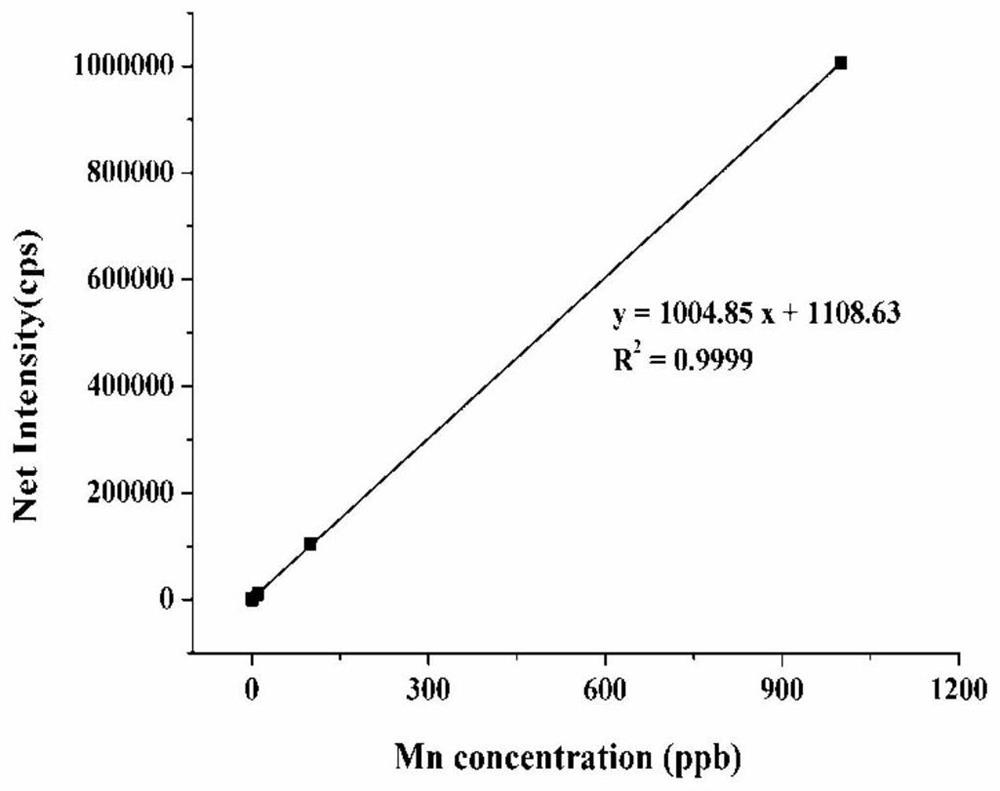
The invention provides a magnetic resonance contrast agent as well as a preparation method and application thereof, and relates to the field of magnetic resonance contrast agents. The magnetic resonance contrast agent disclosed by the invention is prepared from a compound with structural characteristics of a formula I shown in the description. The magnetic resonance contrast agent disclosed by the invention can also be a compound with structural characteristics shown as a formula II or pharmaceutically acceptable salt thereof, M1 is a +2 ion of paramagnetic metal Mn, Fe, Eu or Dy, or a +3 ion of Mn, Fe, Eu or Dy; M2 is Na < + >, K < + > or meglumine cation; when M1 is a +2 valence ion, a is equal to 2; and when M1 is a + 3 valence ion, a is equal to 3. The magnetic resonance contrast agent has the characteristics of good water solubility, high relaxation efficiency, low toxic and side effects and the like.
Owner:GUANGZHOU PINGLAN MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com