Polyionic infusion solution
A multi-ion, solution technology, used in drug delivery, medical preparations with non-active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
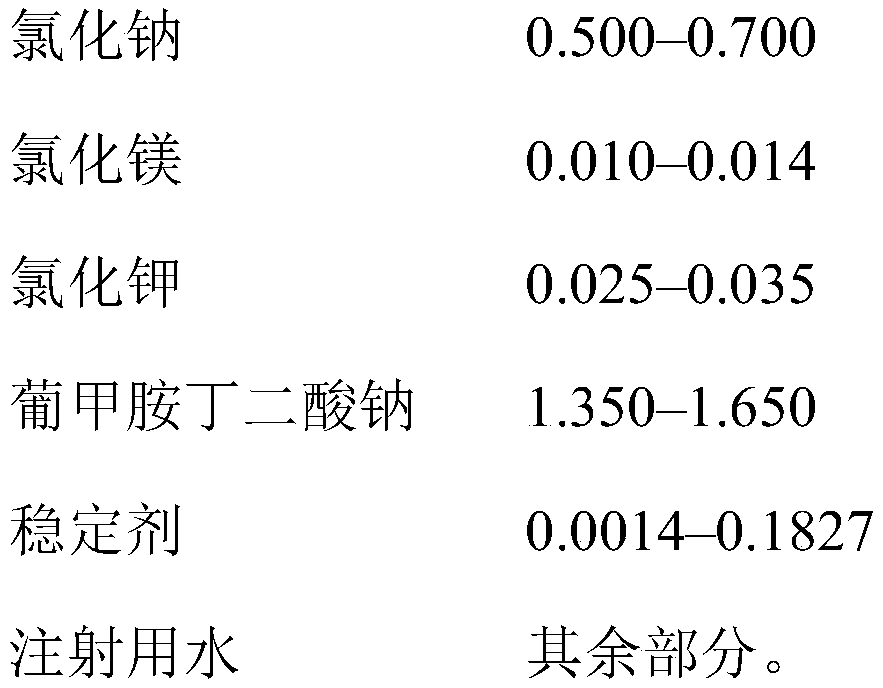
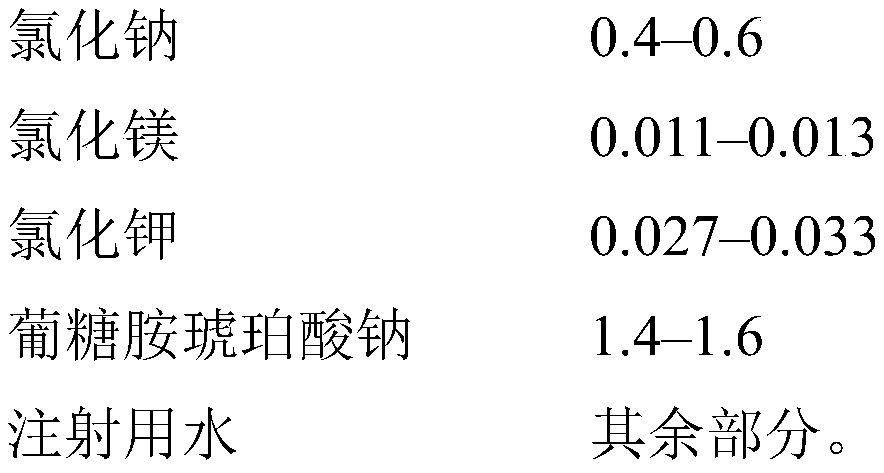
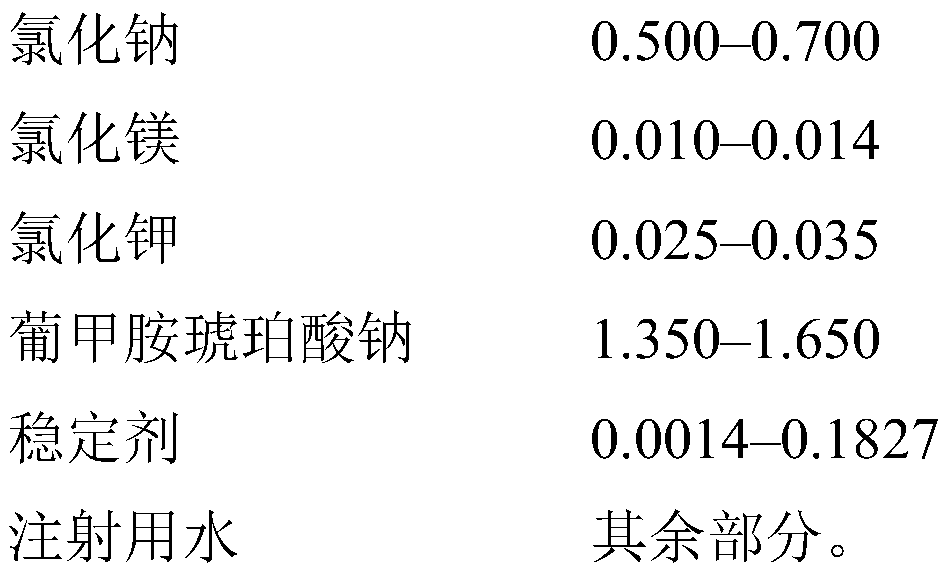
[0053] Example 1. A 1,000-liter mixer tank was charged with 900 liters of water for injection, 5.280 kg of succinic acid, 8.725 kg of meglumine, 1.788 kg of sodium hydroxide, 6.000 kg of sodium chloride, 0.256 kg of magnesium chloride hexahydrate, and 0.300 kg of Potassium chloride, which are mixed until their components are completely dissolved, then brought to a volume of 1,000 liters with water for injection. The resulting solution was sparged with nitrogen for 30 minutes, filtered through a 0.22 μm sterile filter, bottled into 500 ml glass vials and sealed with stoppers under nitrogen flow. Then, undergo 20 minutes of moist heat sterilization at a temperature of 121±1° C. under pressure. 1 liter of the resulting solution contained 5,280 mg of succinic acid (0.528 wt %), 8,725 mg of meglumine (0.872 wt %), 1,788 mg of sodium hydroxide (0.1788 wt %), 6,000 mg of sodium chloride (0.600 wt %), 256 mg of Magnesium chloride hydrate (0.0256 wt%), 300 mg potassium chloride (0.030...
Embodiment 2
[0079] Example 2. A 1,000 liter mixer tank was charged with 900 liters of water for injection, 5.280 kg of succinic acid, 8.725 kg of meglumine, 1.788 kg of sodium hydroxide, 6.000 kg of sodium chloride, 0.256 kg of magnesium chloride hexahydrate, 0.300 kg of chlorine Potassium chloride and 2.0 kg of sodium thiosulfate were mixed until the components were completely dissolved and the pH level was set to a fixed value, then brought to a volume of 1,000 liters with water for injection. The resulting solution was filtered through a 0.22 μm sterile filter, bottled into 500 ml glass vials and sealed with stoppers. Then, undergo 20 minutes of moist heat sterilization at a temperature of 121±1° C. under pressure. 1 liter of the resulting solution contained 5,280 mg of succinic acid (0.528 wt %), 8,725 mg of meglumine (0.872 wt %), 1,788 mg of sodium hydroxide (0.1788 wt %), 6,000 mg of sodium chloride (0.600 wt %), 256 mg of Magnesium chloride hydrate (0.0256 wt%), 300 mg potassium ...
Embodiment 3
[0080] Example 3. A 1,000-liter mixer tank was charged with 900 liters of water for injection, 5.280 kg of succinic acid, 8.725 kg of meglumine, 1.788 kg of sodium hydroxide, 6.000 kg of sodium chloride, 0.256 kg of magnesium chloride hexahydrate, 0.300 kg of Potassium chloride and 0.2 kg of sodium thiosulfate, which were mixed until their components were completely dissolved and the pH level was set to a fixed value, were then brought to a volume of 1,000 liters with water for injection. The resulting solution was filtered through a 0.22 μm sterile filter, bottled into 500 ml glass vials and sealed with stoppers. Then, undergo 20 minutes of moist heat sterilization at a temperature of 121±1° C. under pressure. 1 liter of the resulting solution contained 5,280 mg of succinic acid (0.528 wt %), 8,725 mg of meglumine (0.872 wt %), 1,788 mg of sodium hydroxide (0.1788 wt %), 6,000 mg of sodium chloride (0.600 wt %), 256 mg of Magnesium chloride hydrate (0.0256 wt%), 300 mg potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com