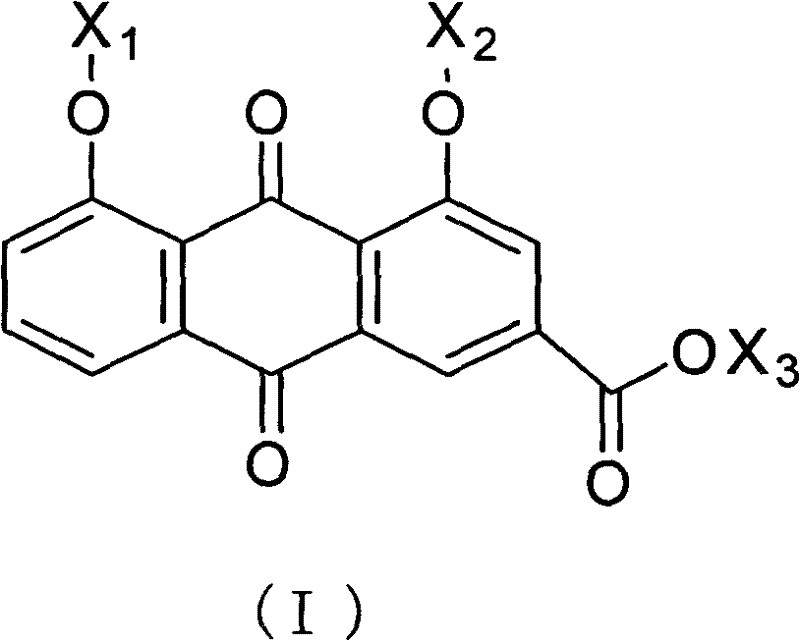
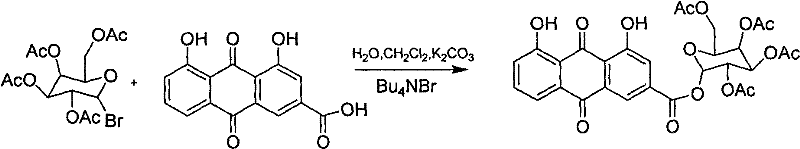Rheinic acid derivatives and treatment application thereof
A technology of rhein and derivatives, applied in the field of medicine, can solve the problems of high pH value of water injection, unacceptable human physiology, poor preparation stability, etc., and achieve obvious pharmacological activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
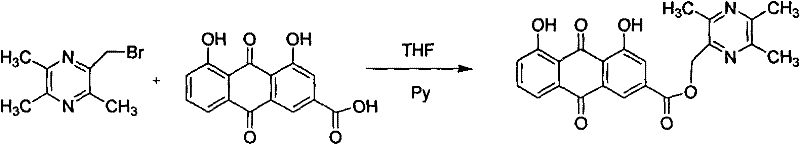
Embodiment 1
[0031] Synthesis of Ligustrazine Rheinate
[0032] 1.1 The synthesis of 2-bromomethyl-3,5,6-trimethylpyrazine (synthesis of bromoligustrazine)
[0033] In a 250ml three-necked flask, sequentially add the raw materials 2,3,5,6-tetramethylpyrazine (20g, 0.147mol), NBS (26.75g, 0.15mol), and benzoyl peroxide (0.05g, 0.0002mol) , The solvent tetrahydrofuran (75mL). The solution was orange-yellow and turbid. Irradiated by incandescent lamp, the oil bath was heated to 75℃, and the reaction was refluxed for 8 hours. TLC [V (ethyl acetate): V (petroleum ether) = 1:2 as the developing solvent] showed that the reaction was complete (raw material) Rf=0.38, product Rf=0.56), the reaction solution was purple-red, the succinimide produced was removed by filtration to obtain purple-red filtrate, the purple-red viscous liquid after tetrahydrofuran was recovered under reduced pressure, and the product was collected by vacuum distillation 99~101℃ / 2mmHg distillate yields 23g of 2-bromomethyl-3,5,6-...
Embodiment 2
[0041] Synthesis of 2,3,4,6-tetraacetylglucose ester of rhein;
[0042] Add rhein (0.68g, 0.0024mol), K 2 CO 3 0.69g, water (10ml), heated to 30°C and stirred to dissolve, the pH of the solution was controlled to be ≈9. Add 10ml of dichloromethane with 0.20g of tetrabutylammonium bromide, continue stirring at 30°C for 30min, and add 10ml of dichloromethane solution of 1.02g (0.0025mol) of 2,3,4,6-tetraacetylglucose bromide dropwise. After the addition is complete, heat to 50°C and stir for 6 hours. TLC shows that the reaction is complete. Separate the organic layer, extract the aqueous layer 3 times with dichloromethane, combine the organic phases, and wash the organic phases with 2% NaOH solution to neutrality. , Dried over anhydrous sodium sulfate, recovered chloroform under reduced pressure, and recrystallized from absolute ethanol to obtain 0.75 g of red solid, with a yield of 51%.
[0043] Reaction formula:
[0044]
[0045] The compound of Example 1 has shown efficacy in xyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com