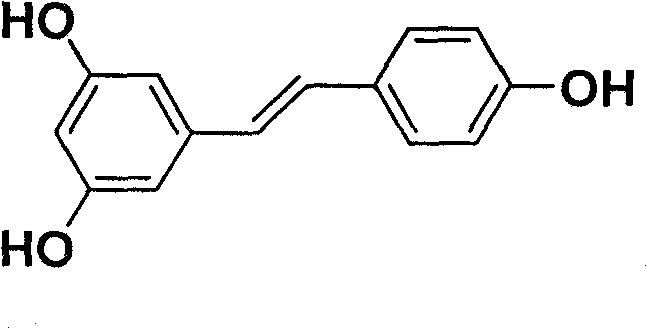
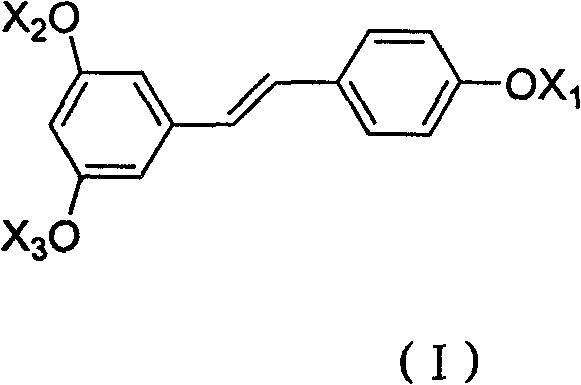
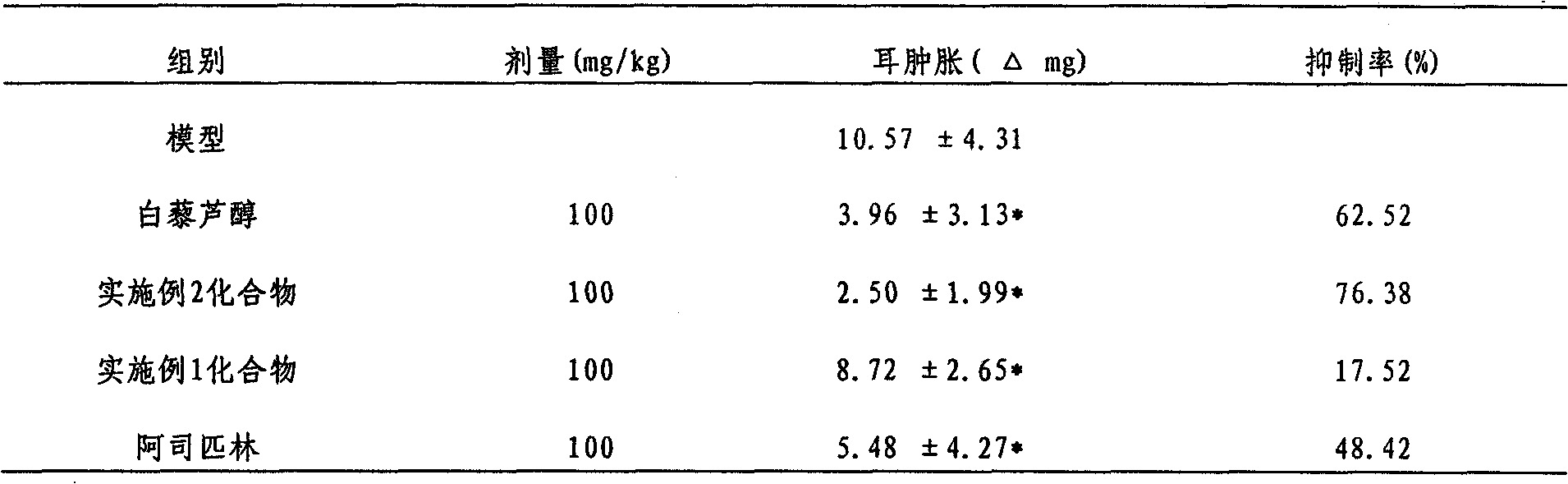
Ether derivative of resveratrol and medical application
A technology of resveratrol and derivatives, applied in the field of medicine, can solve the problems of short-term therapeutic effect, fast biological metabolism, etc., and achieve the effects of convenient medication, prolonged action time, and difficult inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Resveratrol Trioxane
[0039] Take 0.68g (6.24mmol) of bromoethane and potassium carbonate (8.5g, 0.062mol) into a three-necked flask with a stirring device, and add 20ml of DMF. After stirring evenly, add 0.39g of resveratrol (1.71mmol) and 10ml of DMF dropwise. After the dropwise addition, the temperature was raised to 95°C, and the reaction was continued for 5h. After filtering, the filtrate was rotary evaporated under reduced pressure, and the organic solvent was recovered to obtain a yellow solid. Separation by column chromatography yielded 0.32 g of resveratrol trioxane with a yield of 71.2%. 1 H-NMR (CDCl 3 , 300MHz) δ: 7.3 (m, 2H), 6.8 (4H, m), 6.6 (2H, d), 6.2 (1H, t), 4.2-4.3 (6H, m), 1.4-1.5 (9H, m) ; m / e: 312.
Embodiment 2
[0041] Synthesis of Resveratrol Trioxo-3-Methylpyridine
[0042] Weigh resveratrol (3.9g, 0.017mol) and potassium carbonate (8.5g, 0.062mol) into a 250mL three-necked bottle. Add DMF (100mL), heat to 100°C, then add 3-chloromethylpyridine hydrochloride (10.4g, 0.062mol) in portions within 10min, keep the reaction at 95°C, heat the reaction for 4h, and detect the degree of reaction by TLC. After the reaction is basically completed, cool to room temperature, filter the filtrate with suction, add 100mL of water, extract with ethyl acetate 100mL×3, combine the ethyl acetate layers and wash with water (50mL×3), dry with anhydrous sodium sulfate, and depressurize After recovering ethyl acetate, a yellow solid was obtained, which was separated through a silica gel column, and the product was collected. After recovering the solvent under reduced pressure, 6.54 g of resveratrol trioxo-3-methylpyridine was obtained as a light yellow solid, with a yield of 65.1%; 1 H NMR (CDCl 3 , 300M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com