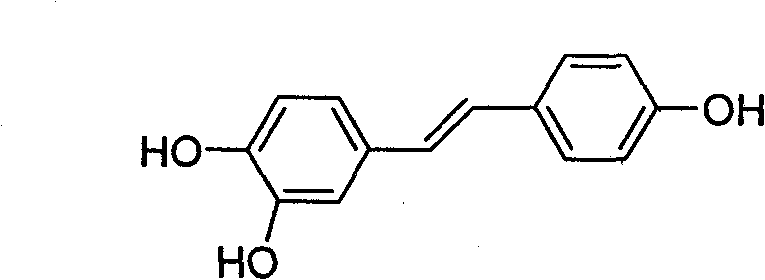
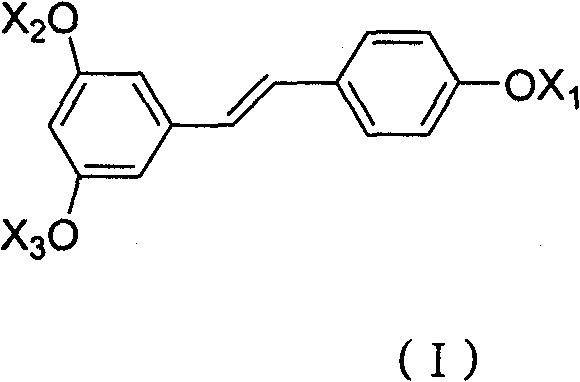
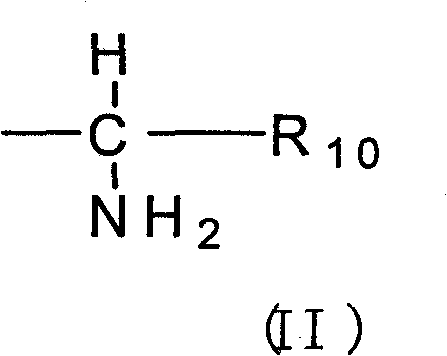Ester derivative of high veratryl alcohol and medical treatment application
A technology of ester derivatives and homoveratrol, which is applied in the field of medicine, can solve the problems of affecting the therapeutic effect and fast biological metabolism, and achieve the effects of reducing the number of doses, slowing down the metabolic rate, and prolonging the drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthesis of Hoveratrol Cinnamon Ester
[0043] 1.1 Synthesis of Cinnamoyl Chloride
[0044] Add 3g (20.24mmol) cinnamic acid into a three-necked flask with a stirring device, add 10ml of dichloromethane, 5-7 drops of N,N-dimethylformamide, stir at room temperature and dropwise add 10ml of SOCl 2 . Stir at room temperature for 2 h, remove SOCl by suction filtration 2 , to obtain a colorless liquid, which was left to cool to obtain 3.2 g of a white solid, with a yield of 94.0%.
[0045] 1.2 Synthesis of homoveratrol cinnamon ester
[0046] Take 1.05g (6.24mmol) of cinnamonyl chloride from the previous reaction and add it to a three-necked flask with a stirring device, and then add 20ml of dry acetone, 20ml of dry Py, and 0.05g of DMAP under stirring at room temperature. After stirring evenly, add 0.39g of homoveratrol (1.73mmol) and 10ml of acetone mixture dropwise. After the dropwise addition, continue to react for 5h, filter, and the filtrate is rotary evaporate...
Embodiment 2
[0048] 2. Synthesis of homoveratrol nicotinate
[0049] 2.1 Synthesis of Nicotinyl Chloride
[0050] Add 3g (24.37mmol) of nicotinic acid into a three-necked flask with a stirring device, add 5-7 drops of N,N-'-methylformamide, 20mL of dichloromethane, stir at room temperature and dropwise add 10mL of SOCl 2 With 20mL dichloromethane mixture. After the dropwise addition, heat to 77°C and reflux for 2h, then remove SOCl by suction filtration 2 , and then add 20 mL of diethyl ether to reflux for 1 h, recover diethyl ether to obtain 4.2 g of white solid, yield 96.2%.
[0051] 2.2 Synthesis of homoveratrol nicotinate
[0052] Take the nicotinyl chloride reacted in the previous step and put it into a three-necked flask with a stirring device, and add 20Ml of dry pyridine under stirring at room temperature. After 5 minutes, add 0.71g (3.12mmol) homoveratrol and 10ml pyridine mixture dropwise. After the dropwise addition, raise the temperature to 75°C, stir for 3h, and recover py...
Embodiment 3
[0054] 3. Synthesis of glycyl homoveratrol
[0055] Add 3g of F-moc glycine and 20ml of oxalyl chloride into a 50ml round bottom flask, heat to 60°C and stir for 3h, remove unreacted oxalyl chloride by rotary evaporation under reduced pressure to obtain a white solid. Add 0.76g homoveratrol, 0.1g DMAP and 20ml pyridine into a 100ml three-necked flask equipped with a stirring device, add 20ml of toluene and the F-moc glycyl chloride mixed solution from the previous step dropwise at room temperature, and heat to 55°C for 6h. Add 20ml of water, extract 3 times with 100ml of ethyl acetate, combine the organic phases, add anhydrous Na 2 SO 4 Let dry overnight. The organic solvent was recovered under reduced pressure to obtain a yellow oily liquid. After separation by silica gel column, 0.61 g of white solid was obtained with a yield of 45%.
[0056] The compounds of Examples 1 and 2 have shown efficacy in xylene-induced mouse ear swelling and 10% egg white-induced rat paw swell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com