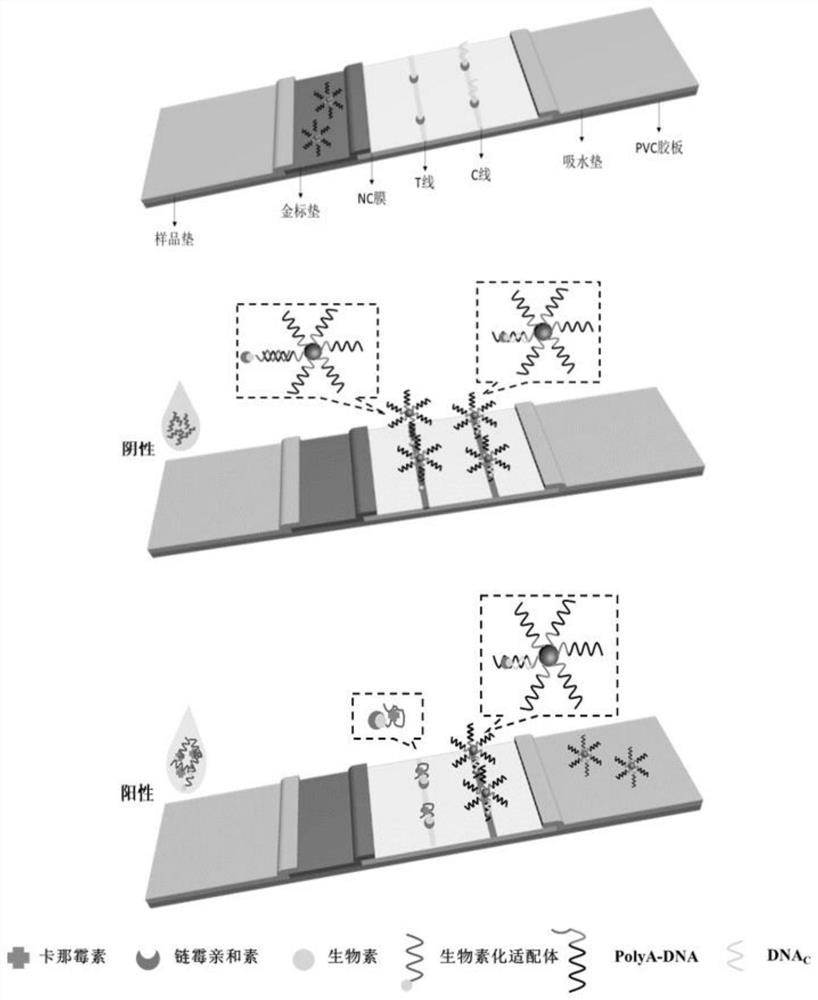
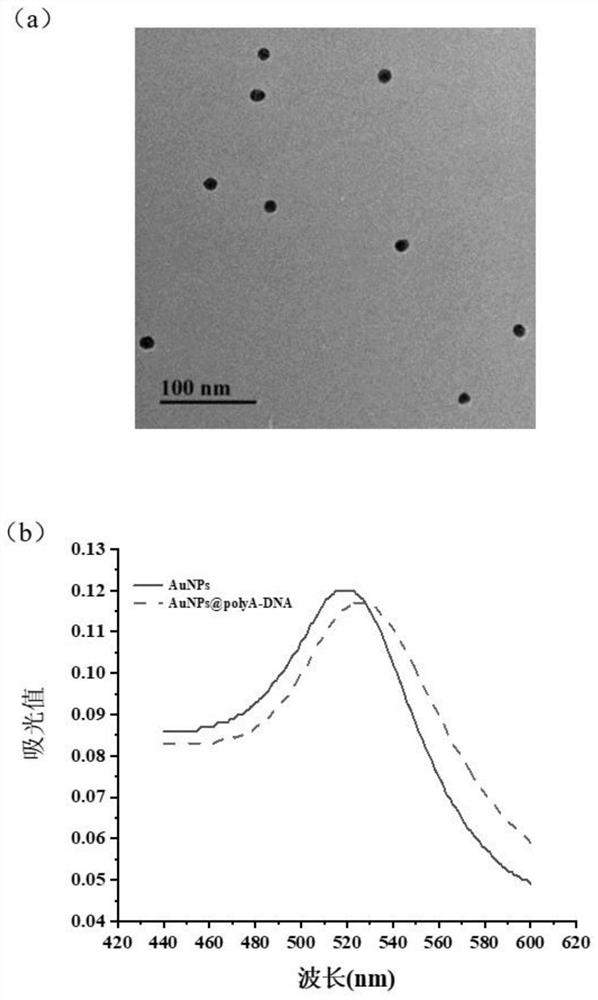
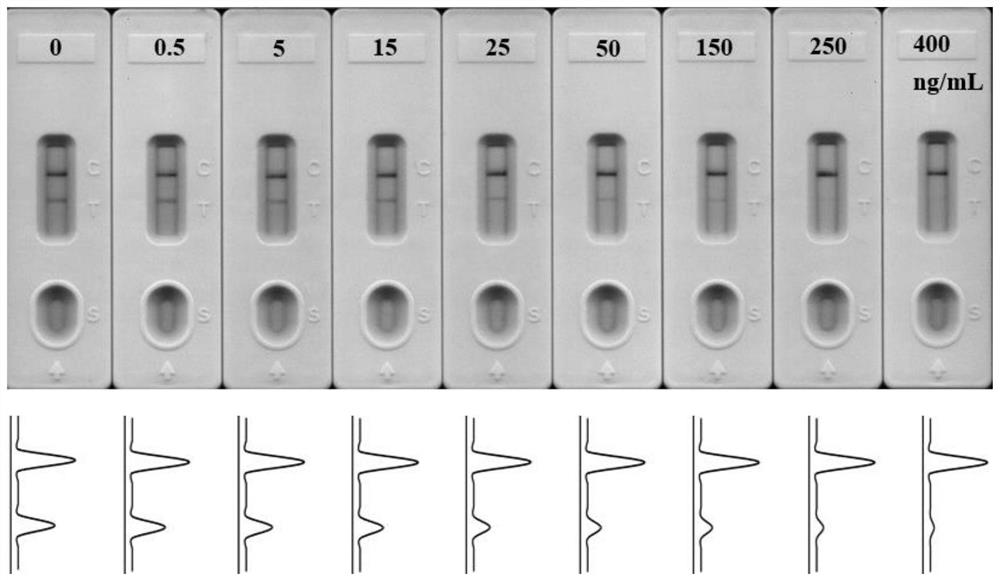
Universal aptamer colloidal gold lateral chromatography test paper for detecting small molecular substances
A technology of small molecular substances and lateral flow chromatography, which is applied in the fields of medicine, environment, food safety detection, nanobiological sensing, and analytical chemistry. It can solve problems such as detection sensitivity needs to be improved, is not conducive to OTA competition, and poor product stability. Achieve the effects of increasing binding efficiency, high repeatability, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of Kanamycin Rapid Detection Test Strips Based on Nucleic Aptamers
[0057] The specific steps are:
[0058] 1. Design of aptamer sequences and probes
[0059] In order to ensure that the biotinylated aptamer can bind to the nucleic acid on the probe and be captured by SA in the T-line region, nucleic acid strands of different sequences (polyA-DNA (20) , polyA-DNA (15) , polyA-DNA (5+10) , polyA-DNA (5+5) , polyA-DNA (10+5) ) coupled with AuNPs (as shown in Table 1). The selected corresponding aptamer concentration was 0.5 μM, and the same concentration of kanamycin (150 ng / mL) was added. The result is as Figure 8 As shown, when multiple T bases are connected at the 3' end of the aptamer sequence, the signal intensity can be significantly increased, and the signal can be enhanced by up to 6 times. When a nucleic acid probe with a nucleotide sequence such as SEQ ID NO.2 is used strand polyA-DNA (10+5) , the change of the corresponding sig...
Embodiment 2
[0091] Embodiment two: with test strip to the mensuration of kanamycin standard solution
[0092] (1) Preparation of Kanamycin Standard Solution
[0093] Kanamycin standard solution was diluted with Runningbuffer (4×SSC, pH 7) to a final concentration of 0.5, 5, 15, 25, 50, 150, 250 and 400 ng / mL. The kanamycin aptamer whose nucleotide sequence is shown in SEQ ID NO.1 was diluted to 0.5 μM with ultrapure water.
[0094] (2) Establishment of standard curve for detection of kanamycin nucleic acid test strips:
[0095] In step (1), 99 μL of kanamycin standard solution of different concentrations and 1 μL of kanamycin aptamer solution were mixed and incubated for 20 min. After the mixed reaction, the mixed liquid was added to the sample pad for detection. After 3 min of reaction, the (T / C) relative signal intensity, and establish a standard curve of the corresponding relationship between (T / C) relative optical signal intensity and different kanamycin concentrations.
[0096] T...
Embodiment 3
[0098] Embodiment three: use test strip to the mensuration of OTA standard solution
[0099] (1) Preparation of OTA standard solution
[0100] The OTA standard solution was diluted with Running buffer (4×SSC, pH7) to a final concentration of 1, 10, 50, 100, 250 and 500 ng / mL. Dilute the OTA aptamer whose nucleotide sequence is shown in SEQ ID NO.1 to 0.5 μM with ultrapure water.
[0101] (2) Establishment of OTA nucleic acid test strip detection standard curve:
[0102] Mix and incubate 99 μL of OTA standard solutions with different concentrations and 1 μL OTA aptamer solution in step (1) for 20 minutes, add the mixed droplets to the sample pad for detection after the mixed reaction, and measure the (T / C) relative signal intensity after 3 minutes of reaction , to establish a standard curve of the corresponding relationship between (T / C) relative optical signal intensity and different OTA concentrations.
[0103] The result is as Figure 5 As shown, when the OTA concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com