Method for removing vanadium citrate in water environment, kit and application
A technology of citric acid and water environment, applied in the field of environmental governance, can solve problems such as difficult and difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, experimental apparatus and method
[0049] 1.1 Experimental reagents and instruments
[0050] (1) The natural iron ore used in the experiment is pyrite, which was purchased in Shiyan, Hubei Province on December 6, 2019.
[0051] The reagents used in the experiment are listed in Table 2, and the water used in the experiment is ultrapure water.
[0052] Table 2 Chemical Reagents
[0053]
[0054]
[0055] Note: The symbol "-" is a standard reagent and does not need to show purity.
[0056] (2) The instruments used in the experiment are listed in Table 3.
[0057] Table 3 Experimental Instruments
[0058]
[0059] 2.2 Experimental scheme
[0060] 2.2.1 Preparation of V(IV)-citric acid solution
[0061] Accurately weigh 0.03196g of vanadyl sulfate and dissolve it in deoxygenated ultrapure water, and let it stand for 48 hours. Weigh 0.0420g of citric acid, stir and dissolve it in ultrapure water, and store it in a brown bottle. Mix the configur...
Embodiment 2
[0092] Embodiment 2, pyrite / PMS removes the performance of vanadium citrate
[0093] 3.1 Effect of initial vanadium citrate concentration on the removal of vanadium citrate
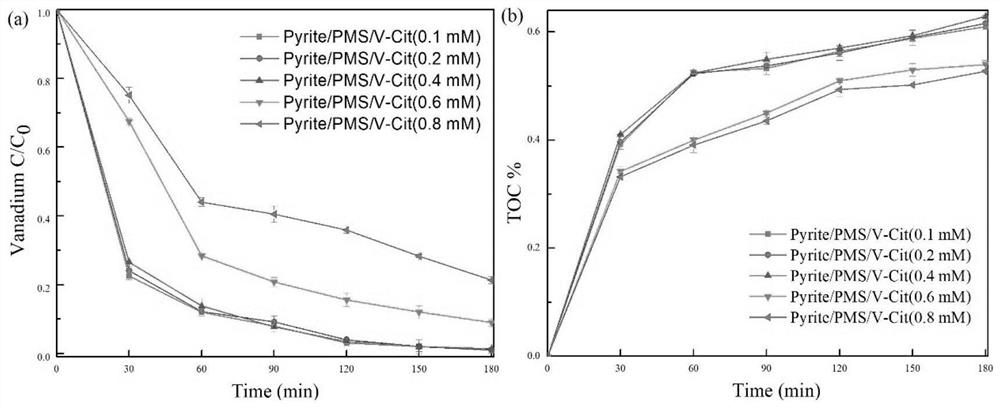
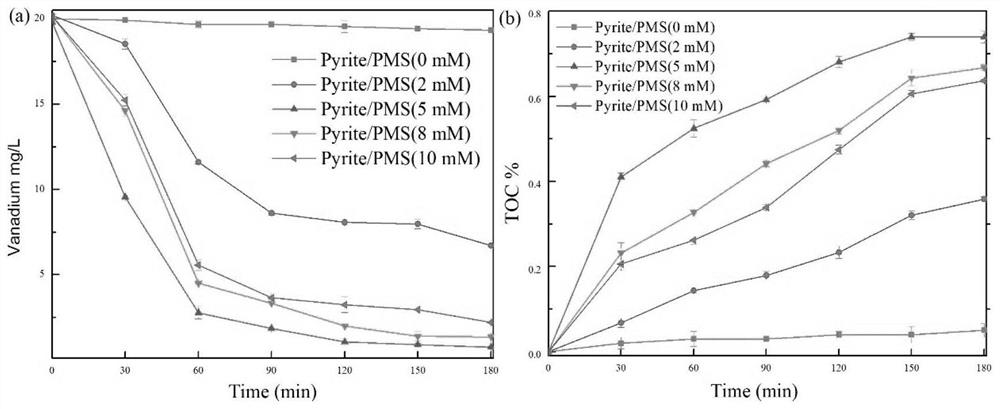
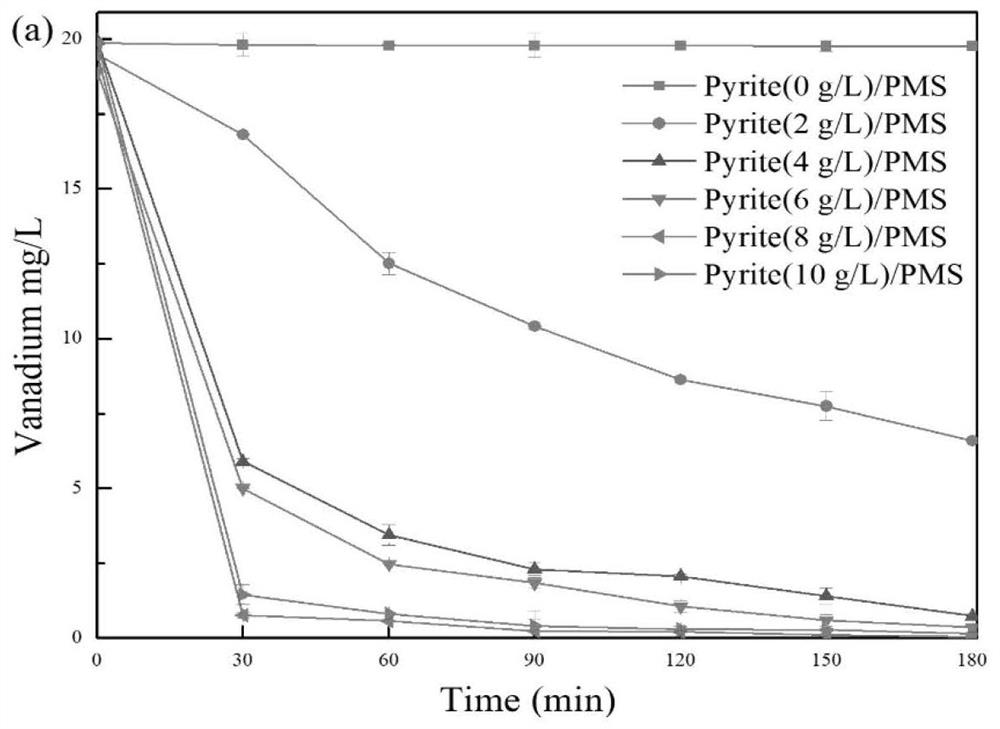
[0094] Under the conditions of PMS concentration of 5mM and pyrite concentration of 8g / L, the effect of the initial vanadium citrate concentration on the reaction system was explored, and the performance of the reaction was measured by the total vanadium removal rate and TOC reduction rate.
[0095] The result is as figure 1 Shown, the reaction performance of 0.1, 0.2, 0.4, 0.6, 0.8mM vanadium citrate solution in pyrite / PMS system. As the initial concentration of vanadium citrate solution increases, within the concentration range of 0.1-0.4mM, vanadium citrate can be degraded rapidly, and the concentration of total vanadium is almost negligible. With the change of V(IV)-citric acid concentration in the reaction system, the degradation rates of total vanadium were 98.9±0.63%, 99.0±0.98%, 99.4±0.87%, 89.7...
Embodiment 4
[0122] Embodiment 4, the mechanism of pyrite / PMS degradation heavy metal complex
[0123] 5.1 Infrared spectrum analysis of heavy metal complexes degraded by pyrite / PMS
[0124] 5.1.1 UV spectrum of pyrite / PMS degradation vanadium citrate system
[0125] Figure 8 It is 8g / L of pyrite, the initial PMS concentration is 5mM, and the initial vanadium citrate concentration is 0.4mM under the condition of reacting, take reaction solution when 0min, 30min, 60min, 90min, 150min, carry out the infrared spectrum obtained by processing and detecting picture. exist Figure 8 , 3400cm also appeared at 0min -1 There is an OH bond attributed to carboxylic acid nearby, and the peak maintains a wide distance, which proves that V(IV) has a high degree of association with the organic ligand, and the heavy metal organic complex exists in a stable form. After 30 minutes of reaction, 3400cm -1 The peak tends to be horizontal, and in the first 30 minutes of the reaction, rapid and efficient d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com