Brominated hydrogenated nitrile rubber as well as preparation method and application thereof
A technology of hydrobrominated nitrile and nitrile rubber, which is applied in the field of rubber, can solve the problems of poor low temperature resistance and poor flexibility of molecular chains, and achieve the effects of improving low temperature resistance, increasing production efficiency, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The present embodiment provides a kind of preparation method of hydrobromated nitrile rubber, and the steps are as follows:
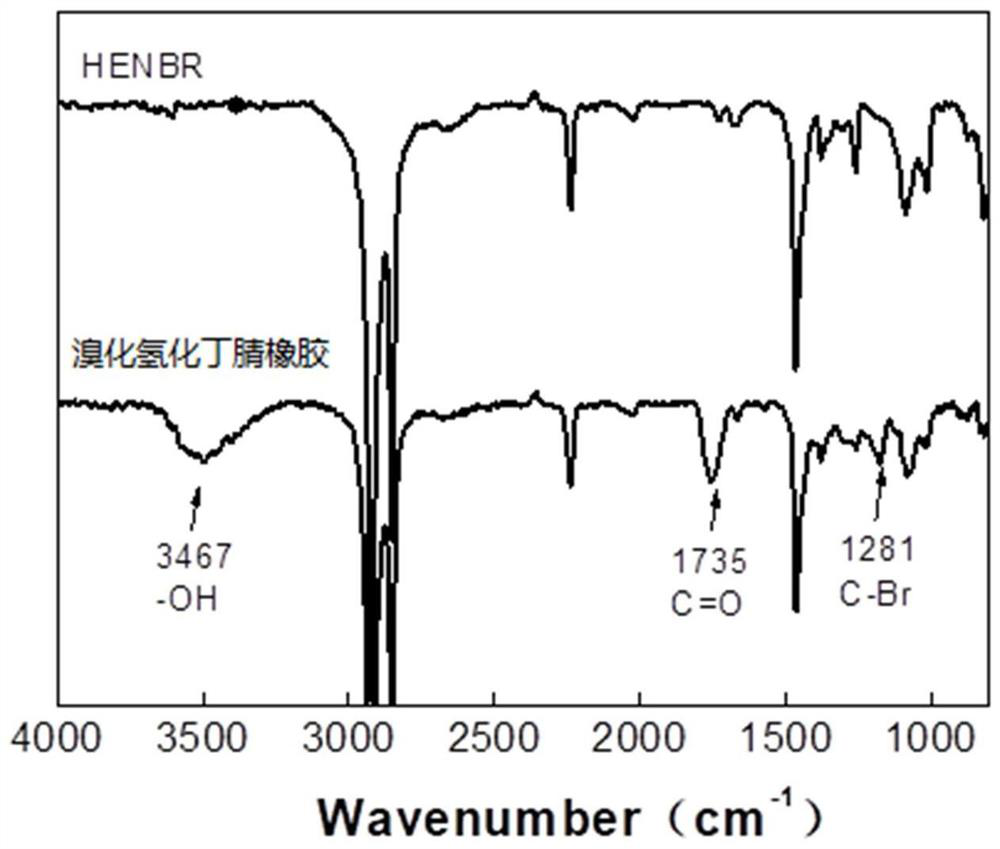
[0044] Epoxidation reaction: Dissolve 1mol nitrile rubber in organic solvent chlorobenzene to make a solution with a mass fraction of 8%, add 0.2mol acetic acid and 0.4mol hydrogen peroxide, and perform epoxidation reaction at 60°C for 6h, and use the obtained product Dry after ethanol precipitation, obtain the epoxidized nitrile rubber precursor (abbreviation ENBR) that degree of epoxidation is 20%;
[0045] Hydrogenation reaction: dissolve it in chlorobenzene to make 3% ENBR glue, carry out hydrogenation reaction in the reaction kettle through high temperature, high pressure and catalysis, and obtain the epoxidized hydrogenated nitrile rubber precursor with a hydrogenation degree of about 95% (referred to as HENBR).
[0046] Wherein, the catalyst is Wilkinson catalyst and ligand triphenylphosphine.
[0047] The conditions of the hydrogenation...
Embodiment 2
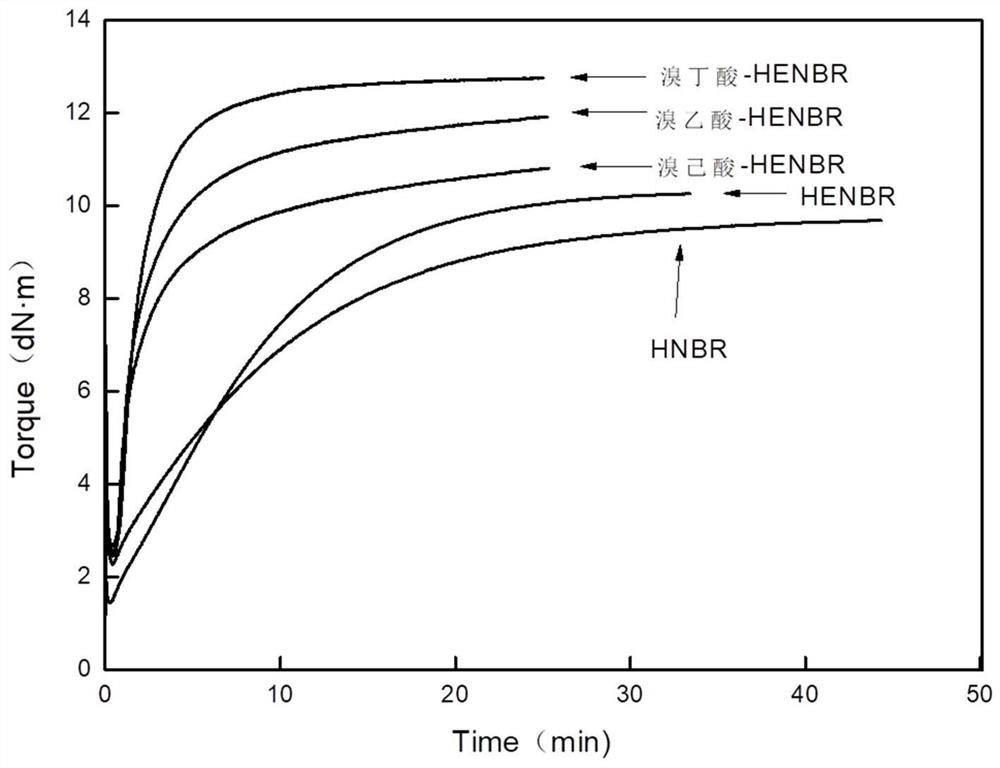
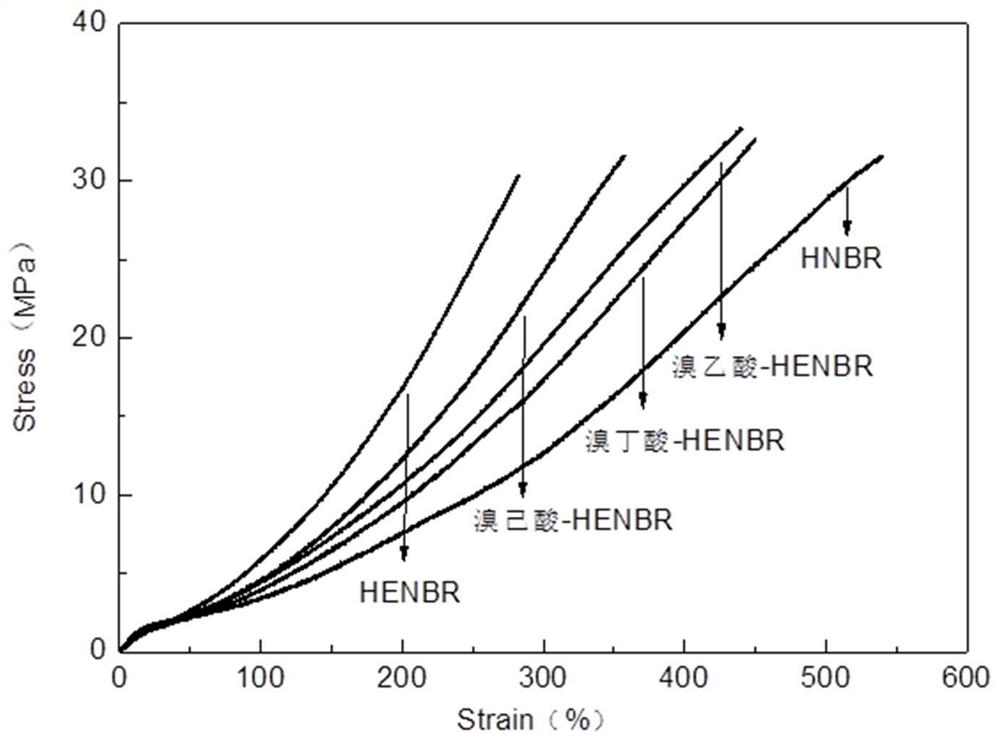
[0051] This embodiment provides a preparation method of hydrogen brominated nitrile rubber, the difference from the preparation method of Example 1 is that the ring-opening reagent is replaced by bromobutyric acid (bromobutyric acid-HENBR for short).
Embodiment 3
[0053] This embodiment provides a preparation method of hydrogen brominated nitrile rubber, the difference from the preparation method of Example 1 is only that the ring-opening reagent is replaced with bromohexanoic acid (bromohexanoic acid-HENBR for short).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com