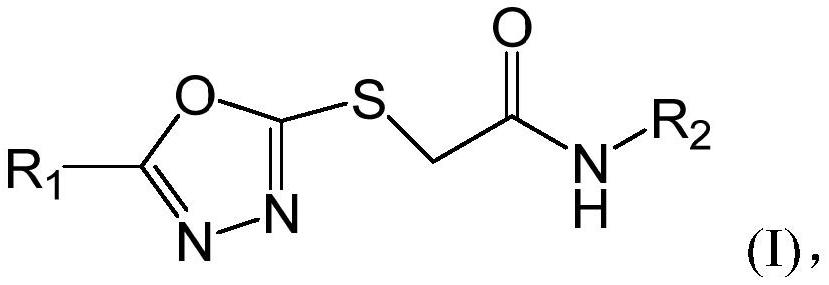
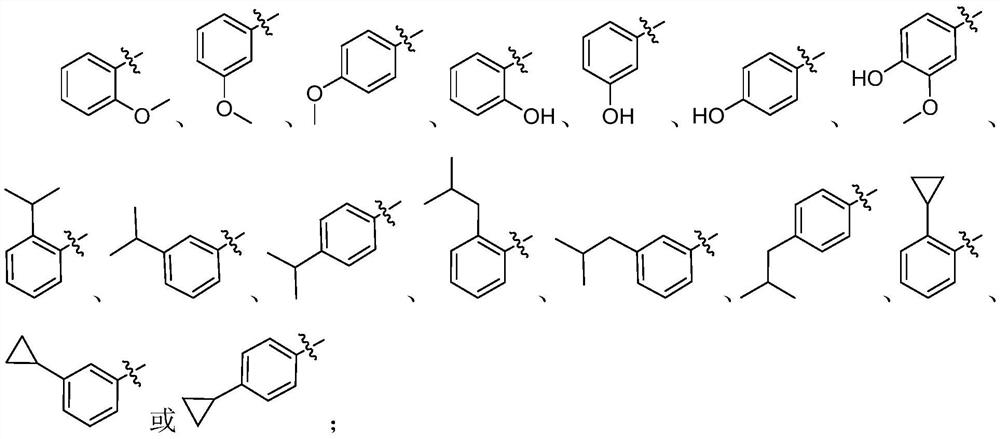
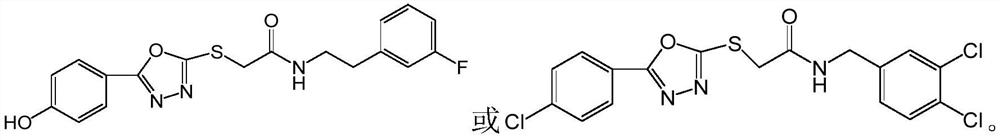
1, 3, 4-oxadiazole neuraminidase inhibitor as well as preparation method and application thereof
A technology of neuraminidase and oxadiazoles, which is applied in the field of biomedicine, can solve the problems of expensive raw materials and complex synthesis process of Tamiflu, and achieve good neuraminidase inhibitory activity, simple synthesis method and excellent neuraminidase. Effect of Acidase Inhibitory Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Described preparation method specifically comprises the following steps:
[0052] (1) After aniline, chloroacetyl chloride and triethylamine are reacted, the intermediate of formula (II) is obtained through aftertreatment;
[0053] (2) benzohydrazide, carbon disulfide and potassium hydroxide are reacted to obtain the intermediate of formula (III) through aftertreatment;
[0054] (3) dissolving the intermediates of formula (II) and intermediates of formula (III) in an organic solvent, and post-processing after the reaction to obtain the 1,3,4-oxadiazole inhibitors shown in formula (I);
[0055] In step (1), triethylamine is used as a catalyst, the organic solvent is dichloromethane, the temperature of the reaction system is 0-20°C, preferably 15°C, and the reaction time is 8-24h, preferably 24h, the post-treatment process is as follows: After the reaction is completed, use 1moL -1Hydrochloric acid solution, saturated sodium bicarbonate solution and saturated brine wash...
Embodiment 1
[0061] A preparation method of 1,3,4-oxadiazole neuraminidase inhibitor, its structural formula is as shown in formula I:
[0062]
[0063] Concrete synthetic steps are as follows:
[0064] (1) Accurately weigh 0.7g (5mmol) of 3-fluorophenethylamine and 0.51g (5mmol) of triethylamine in a 250ml round bottom flask, pour 30ml of dichloromethane into it, protect it under nitrogen, and stir in an ice bath, Then, 0.37 mL (5 mmol) of chloroacetyl chloride was added dropwise with a 1 ml disposable syringe. After the dropwise addition was completed, the reaction was stirred at 15°C for 24 hours. After the reaction was completed, 1 moL -1 Wash with hydrochloric acid solution, saturated sodium bicarbonate solution and saturated brine, dry the organic phase with anhydrous sodium sulfate, and remove the solvent in vacuo to obtain the intermediate of formula (II).
[0065] (2) Accurately weigh 0.76g (5mmol) of 4-hydroxybenzoic hydrazide, 0.28g (5mmol) of potassium hydroxide in a 250ml...
Embodiment 2
[0096]A preparation method of 1,3,4-oxadiazole neuraminidase inhibitor, its structural formula is as follows, and it is prepared by a method similar to Example 1.
[0097] 2-(N-(3,4-dichlorobenzyl)carbonylmethylenemercapto)-5-(4-chlorophenyl)-1,3,4-oxadiazole
[0098]
[0099] Pale yellow solid, yield 35%, IC 50 The value is 0.032 μM.
[0100] 1 H NMR (500MHz, DMSO-d 6 )δ8.92(t, J=6.1Hz, 1H), 7.93(d, J=8.2Hz, 2H), 7.64(d, J=8.2Hz, 2H), 7.50(d, J=9.5Hz, 2H) ,7.24(d,J=8.4Hz,1H),4.32(d,J=6.0Hz,2H),4.18(s,2H). 13 C NMR (125MHz, DMSO-d 6 )δ166.88, 164.79, 164.08, 140.70, 137.19, 131.38, 130.83, 130.00, 129.63, 128.54, 128.03, 122.28, 42.03, 35.98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com