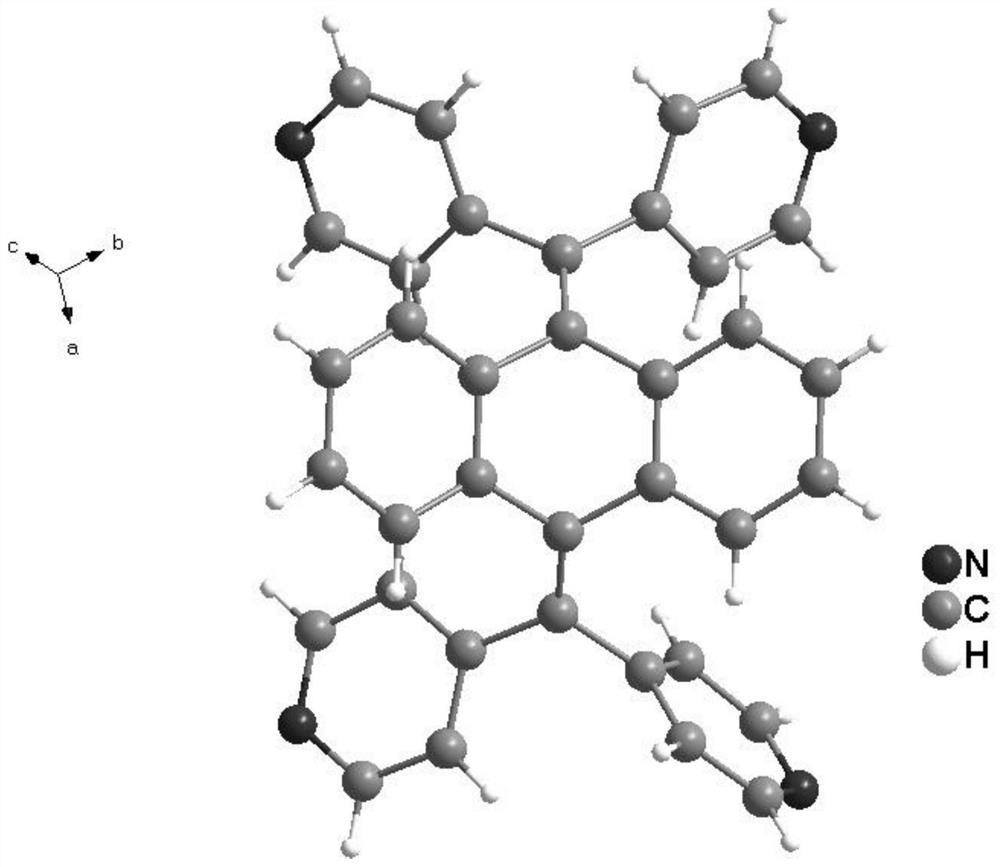
Preparation method of 9, 10-bis (di(pyridine-4-yl) methylene)-9, 10-dihydroanthracene
A technology of dibromomethylene and 10-, which is applied in the field of preparation of organic compounds, achieves the effect of wide application value and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 9,10-bis(dibromomethylene)-9,10-dihydroanthracene, 4-pyridineboronic acid, anhydrous sodium carbonate, triphenylphosphine into 500ml 1,4-dioxane / H 2 O (V:V=4 / 1) mixed solvent, after heating to 120° C. under nitrogen atmosphere, palladium acetate was added to react for 24 hours.
[0034] After the reaction solution was cooled to room temperature, it was transferred to a separatory funnel and added with 300ml of water to shake well, then 200ml of ethyl acetate was added to extract three times, the organic phases were combined and dried with anhydrous sodium sulfate, filtered and rotary evaporated under reduced pressure.
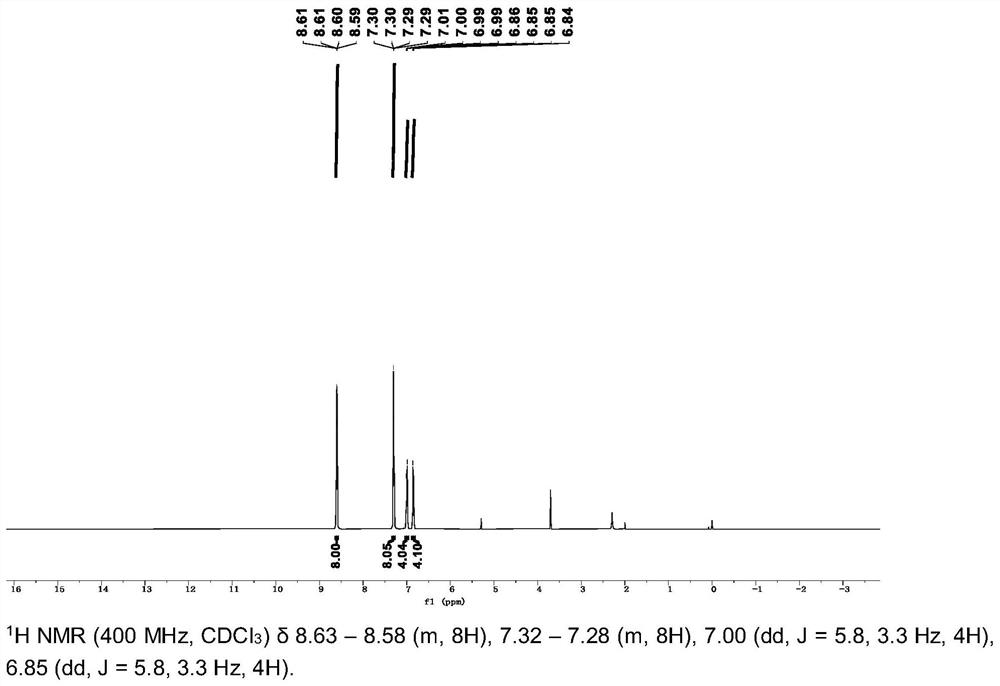
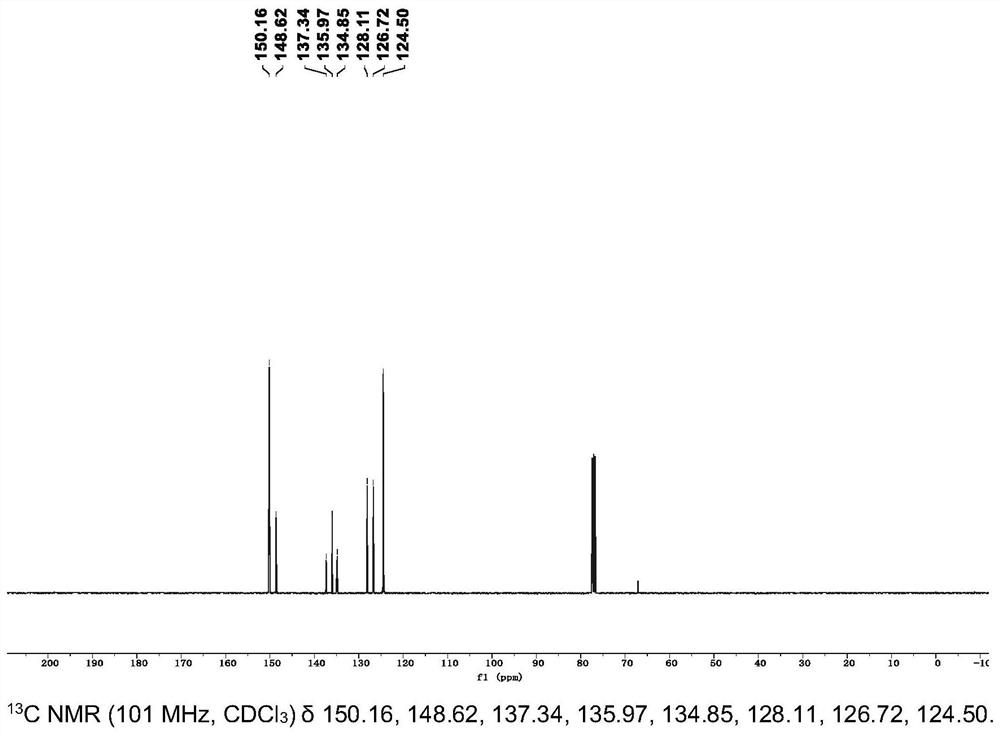
[0035] The crude product was obtained by flash column chromatography using methanol / dichloromethane (V / V=1:10) as the eluent, and 3 g of the crude product was added to 150 ml of acetonitrile, heated and stirred until boiling and then filtered while hot, and this operation was repeated for 3 Once, the product white solid powder 9,10-bis(bis(pyridin-4-...
Embodiment 2
[0041] 9,10-bis(dibromomethylene)-9,10-dihydroanthracene (5.2g, 10mmol), 4-pyridineboronic acid (9.8g, 80mmol), anhydrous sodium carbonate (10.6g, 100mmol), Triphenylphosphine (1.3g, 5mmol) was added to 500ml 1,4-dioxane / H 2 O (V:V=4 / 1) mixed solvent, after heating under nitrogen atmosphere, add palladium acetate to react, the reaction temperature and reaction time are shown in Table 2.
[0042] After the reaction solution was cooled to room temperature, it was transferred to a separatory funnel and added with 300ml of water to shake well, then 200ml of ethyl acetate was added to extract three times, the organic phases were combined and dried with anhydrous sodium sulfate, filtered and rotary evaporated under reduced pressure.
[0043] The crude product was obtained by flash column chromatography using methanol / dichloromethane (V / V=1:10) as the eluent, and 3 g of the crude product was added to 150 ml of acetonitrile, heated and stirred until boiling and then filtered while hot...
Embodiment 3
[0048] 9,10-bis(dibromomethylene)-9,10-dihydroanthracene (5.2g, 10mmol), 4-pyridineboronic acid (9.8g, 80mmol), anhydrous sodium carbonate (10.6g, 100mmol), Triphenylphosphine (1.3g, 5mmol) was added to 1,4-dioxane / H 2 In a mixed solvent of O, after heating to 120°C under a nitrogen atmosphere, add palladium acetate to react for 24 hours; where 1,4-dioxane / H 2 The volume and volume ratio of O mixed solvent are shown in Table 3.
[0049] After the reaction solution was cooled to room temperature, it was transferred to a separatory funnel and added with 300ml of water to shake well, then 200ml of ethyl acetate was added to extract three times, the organic phases were combined and dried with anhydrous sodium sulfate, filtered and rotary evaporated under reduced pressure. The crude product was obtained by flash column chromatography using methanol / dichloromethane (V / V=1:10) as the eluent, and 3 g of the crude product was added to 150 ml of acetonitrile, heated and stirred until b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com