Recombinant amide hydrolase gene and application thereof
An amidohydrolase and gene technology, which is applied to recombinant amidohydrolase genes and their application fields, can solve problems such as lack of an amidohydrolase preparation method, and achieve the effects of simple and efficient preparation method, improved expression efficiency and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment provides a recombinant amidohydrolase gene, which includes the nucleotide sequence shown in SEQ ID No.1.
[0056] SEQ ID No. 1:
[0057] atgctgtctctggttctgccgggtatcggtcacgctcagaccacccagccgccgccggctccggctaaaccggttctgttcaccaacttccgtctgttcgacggtaaatcttctgctctgcgtgacggtctgtacatggttgttgaaggtaacaccatctctcagatcggtcagggtcagccggcttcttctgaaggtaaaaccgttgttgactgcggtggtaaagttatcatgccgggtctgatcgacatgcactggcactctctgctggctgctctgccgatccaggttatcctgcaggctgacatcgctttcgttcacctggctgcttctgctgaagctgaacgtaccctgatgcgtggtttcaccaccatccgtgacgctggtggtccgtctttcgctctgaaacaggctatcgactctggtatgatctctggtccgcgtatctacccgtctggtgctatgatcaccaccaccggtggtcacggtgacttccgttctctggctgaactgccgcgtacctctaaccaggtttctcaggctgaacgtgacggtgctaacgctatcgctgacaccgctgacgaagttcgtatgcgtgttcgtgaacagttcatccagggtgctacccagatcaaaatggttggttgcggtggtgtttctaccccgcgttctccgctggacatgctgaccttcaccgaagaccagatgcgtgctgctgttgaaaccgctgctgactggggtacctacatcctggttcacgcttacaccccggaatctatccagcgttctgttgctgctggtgttcagtgcgttgaacacggtcacctgatgga...
Embodiment 2
[0060] This embodiment provides a recombinant vector, which is a recombinant pET-21a vector containing the recombinant amidohydrolase gene in Example 1.
[0061] After GenScript synthesized the recombinant amidohydrolase gene fragment, it was connected to the pET-21a vector to obtain the recombinant pET-21a vector.
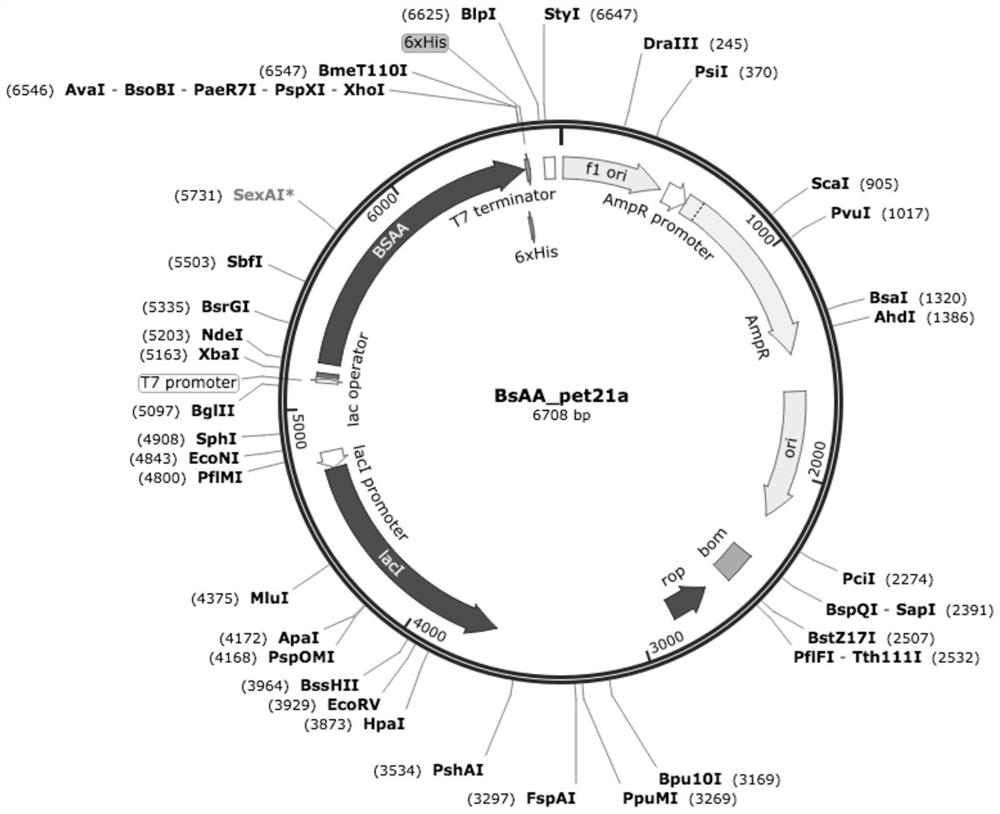
[0062] The plasmid map of the recombinant vector is as follows figure 1 shown.
Embodiment 3
[0064] This embodiment provides a recombinant cell, which is a competent cell containing the recombinant vector in Embodiment 2.
[0065] The recombinant cells are prepared by the following method:
[0066] (1) Thaw ArcticExpress DE3, OverExpress C43 DE3, ER2566HE and BL21 DE3 competent cells on ice, add recombinant vector, mix gently, and ice bath for 25 minutes;
[0067] (2) After heat shock at 42°C for 45s, ice bath for 2min;
[0068] (3) Add 700 μL of LB medium without antibiotics to the tube, and incubate with shaking at 37°C and 200 rpm for 60 min;
[0069] (4) The cultured bacterial solution is spread on the surface of LB solid medium containing 0.1g / mL ampicillin, and placed upside down in an incubator for 14 hours;
[0070] (5) A single colony grown on the plate is a positive clone, and the recombinant cells are obtained after identification and screening.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com