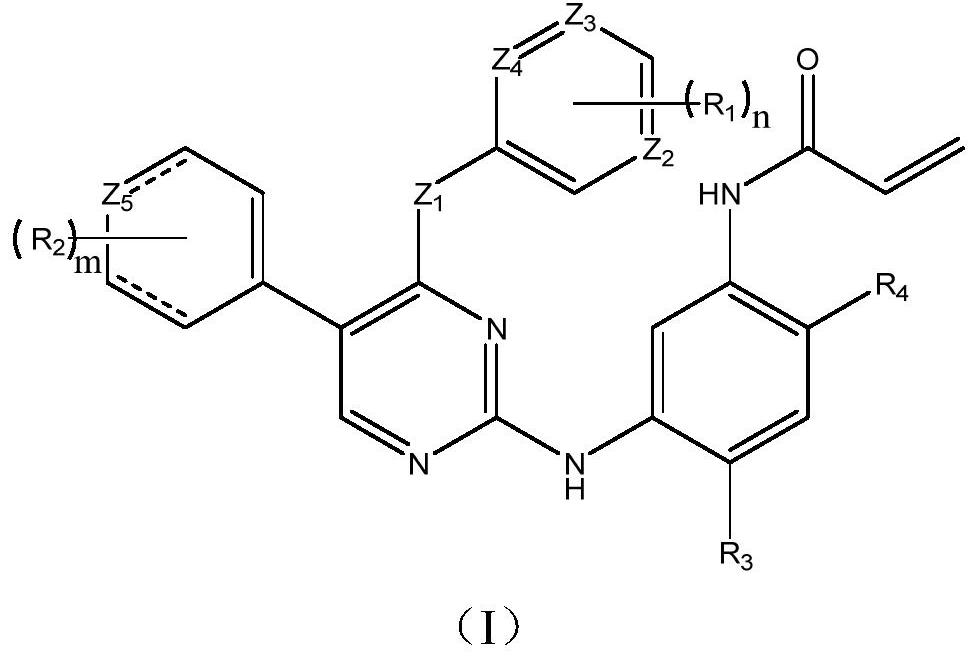
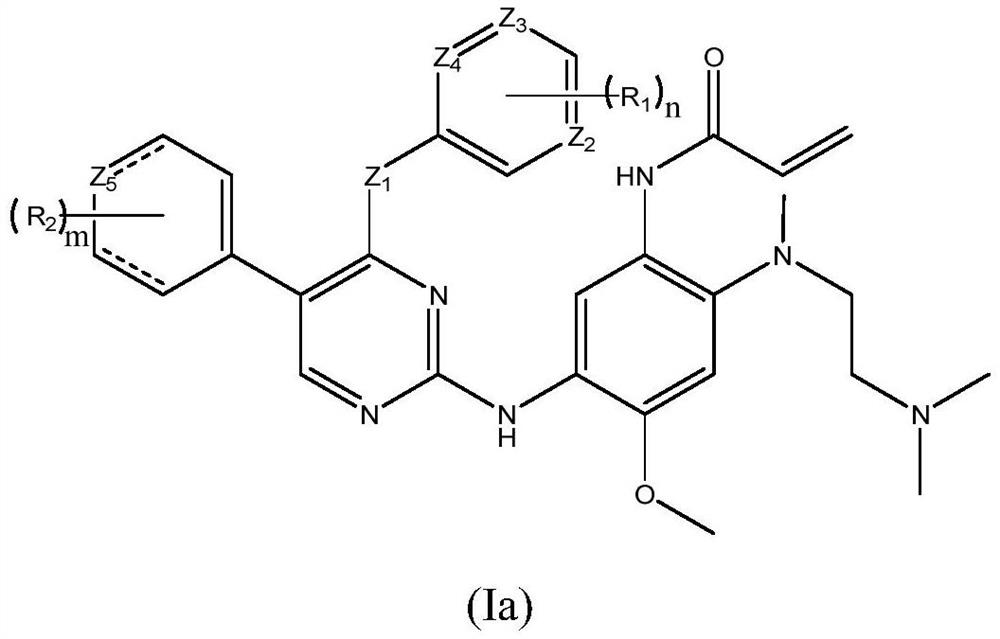
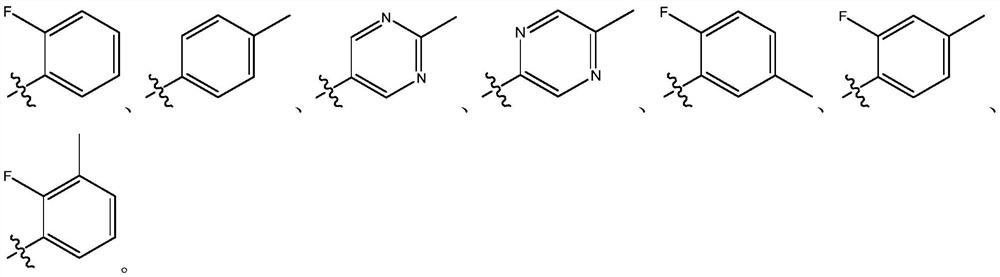
EGFR tyrosine kinase inhibitor and application thereof
An amino, selected technology, applied in the field of chemical medicine, can solve the problems of dose-dependent toxicity, no further confirmation of clinical effect, poor effect of drug-resistant T790M mutant, and achieves good prognosis effect, improved curative effect, and good inhibition. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-((2-fluorophenyl)amino)-5-(4- Methoxyphenyl)pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide
[0047]
[0048] step one:
[0049]
[0050] Compound 1.1 (15.000g, 54.57mmol, 1eq.) was dissolved in DMSO (100mL), and compound 1.2 (6.67g, 60.03mmol, 1.1eq.) was added, K 2 CO 3 (15.08g, 109.14mmol, 2eq.), heated to 80°C, and reacted for 15 hours. Pour the system into water (200 mL), let stand for 30 min, filter, and dry the filter cake to obtain a brown solid (7.350 g, yield 38.53%).
[0051] Step two:
[0052]
[0053] Compound 1.3 (2.500g, 7.15mmol, 1eq.) was dissolved in dioxane (100mL), compound 1.4 (1.30g, 8.58mmol, 1.2eq.), Pd(dppf)Cl 2 (523.35mg, 715.25umol, 0.1eq.), K 3 PO 4 (1.52g, 7.15mmol, 1eq.), H 2 O (10mL), replaced by nitrogen three times, protected by nitrogen, heated to 60°C, reacted for 1.5 hours, poured the system into water (150mL), extracted with EtOAC (300mL), separated the li...
Embodiment 2
[0066] Example 2 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((5-(4-fluorophenyl)-4-(p-tolylamino) Pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide
[0067]
[0068] Using a method similar to Example 1, replace compound 1.2 in step 1 with p-toluidine, and compound 1.4 in step 2 with (4-fluorophenyl)boronic acid to obtain the title compound. 1 H NMR (400MHz, DMSO-d6) (ppm): 10.13 (s, 1H), 8.60 (s, 1H), 7.96 (s, 1H), 7.86 (s, 1H), 7.84 (s, 1H), 7.47- 7.50(m,2H),7.42-7.45(d,2H),7.28-7.32(t,2H),6.94-6.98(m,3H),6.40-6.44(m,1H),6.18-6.22(dd,1H ),5.73-5.76(dd,1H),3.80(s,3H),2.85-2.88(t,2H),2.71(s,3H),2.23-2.31(t,2H),2.18-2.22(m,9H ). LC-MS(m / z):569.69[M+H] + .
Embodiment 3
[0069] Example 3 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((5-(4-fluorophenyl)-4-((2-methyl Pyrimidin-5-yl)amino)pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide
[0070]
[0071] Using the method similar to Example 1, the compound 1.2 in step 1 was replaced by 2-methylpyrimidin-5-amine, and the compound 1.4 in step 2 was replaced by (4-fluorophenyl)boronic acid to obtain the title compound. 1 H NMR (400MHz, DMSO-d6) (ppm): 10.08 (brs, 1H), 8.84 (s, 2H), 8.55 (brs, 1H), 8.37 (s, 1H), 8.12 (s, 1H), 7.93 ( s,1H),7.50-7.54(m,2H),7.29-7.34(t,2H),6.97(s,1H),6.34(s,1H),6.14-6.18(d,1H),5.70-5.73( m, 1H), 3.79(s, 3H), 2.91-2.93(m, 2H), 2.71(s, 3H), 2.28-2.50(m, 9H). LC-MS(m / z):571.66[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com