Preparation method of N-(2-aminoethyl) glycine derivative
A technology for glycine and derivatives, which is applied in the field of preparation of N-glycine derivatives, can solve the problems of difficult tracking, identification and purification, complex operation and purification, and low production yield, and achieve optimal reaction condition parameters, cheap and easy-to-obtain raw materials, and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
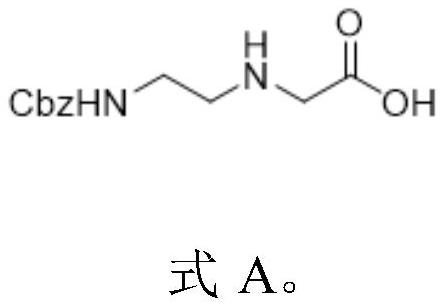
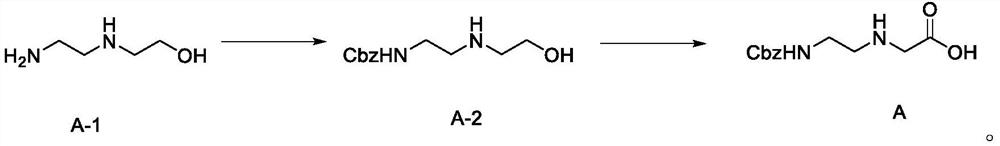
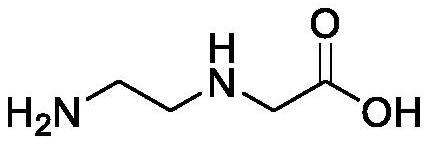
[0084] Embodiment 1, the preparation of formula A compound
[0085] step 1,
[0086] Compound A-1 (1.04g, 10mmol) was added to 20ml of tetrahydrofuran, then sodium bicarbonate (1.6g, 20mmol) and 20ml of water were added, and Cbz-Osu (3g, 12mmmol) was slowly added under stirring for about 30min to maintain the pH ≈8. After the addition, the system was stirred at room temperature until the reaction was complete, concentrated to remove THF, extracted with ethyl acetate, dried and concentrated the organic phase to obtain product A-2 (1.9 g, yield 80%).
[0087] Step 2,
[0088] A solution of A-2 (0.95 g, 4 mmol) in 40 mL of acetone was added to 15 mL of saturated sodium bicarbonate solution. Cool down to below 5°C with an ice bath, add NaBr (0.1g, 1mmol), TEMPO (0.015g, 0.1mmol), then sodium hypochlorite (0.6g, 8.0mmol), sodium chlorate (0.875g, 8.0mmol) in batches Add, keep the system temperature below 5°C, stir until the reaction is complete, add isopropanol and stir for 30 m...
Embodiment 2
[0089] Embodiment 2, the preparation of formula A compound
[0090] step 1,
[0091] Compound A-1 (1.04g, 10mmol) was added to 14ml of tetrahydrofuran, then sodium bicarbonate (1.9g, 25mmol) and 16ml of water were added, and Cbz-Osu (2.9g, 11mmmol) was slowly added under stirring for about 30min, and maintained pH≈8. After the addition, the system was stirred at room temperature until the reaction was complete, concentrated to remove THF, extracted with ethyl acetate, dried and concentrated the organic phase to obtain product A-2 (1.95 g, yield 82%).
[0092] Step 2,
[0093] A solution of A-2 (0.95 g, 4 mmol) in 30 mL of acetone was added to 15 mL of saturated sodium bicarbonate solution. Cool down to below 5°C with an ice bath, add NaBr (0.133g, 133mmol), TEMPO (0.019g, 0.133mmol), then sodium hypochlorite (0.67g, 9.0mmol), sodium chlorate (0.98g, 9.0mmol) in batches Add, keep the system temperature below 5°C, stir until the reaction is complete, add isopropanol and stir ...
Embodiment 3
[0094] Embodiment 3, the preparation of formula A compound
[0095] step 1,
[0096] Compound A-1 (1.04g, 10mmol) was added to 14ml of tetrahydrofuran, then sodium bicarbonate (2.4g, 30mmol) and 21ml of water were added, and Cbz-Osu (3.3g, 15mmmol) was slowly added under stirring for about 30min, and maintained pH≈8. After the addition, the system was stirred at room temperature until the reaction was complete, concentrated to remove THF, extracted with ethyl acetate, dried and concentrated the organic phase to obtain product A-2 (2.01 g, yield 85%).
[0097] Step 2,
[0098]A solution of A-2 (0.95 g, 4 mmol) in 45 mL of acetone was added to 15 mL of saturated sodium bicarbonate solution. Cool down to below 5°C with an ice bath, add NaBr (0.08g, 0.08mmol), TEMPO (0.012g, 0.08mmol), then sodium hypochlorite (0.75g, 10.0mmol), sodium chlorate (1.09g, 10.0mmol) Add in batches, keep the system temperature below 5°C, stir until the reaction is complete, add isopropanol and stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com