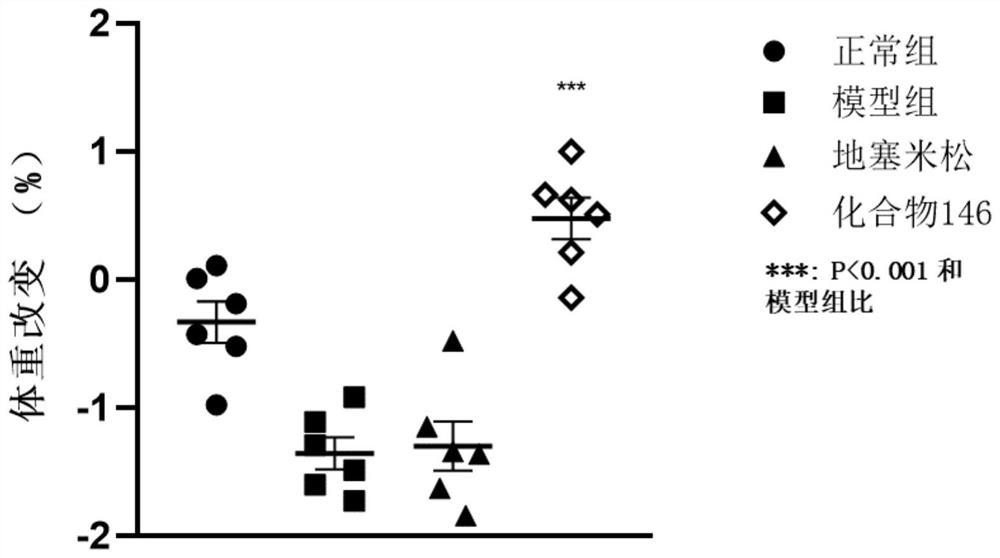
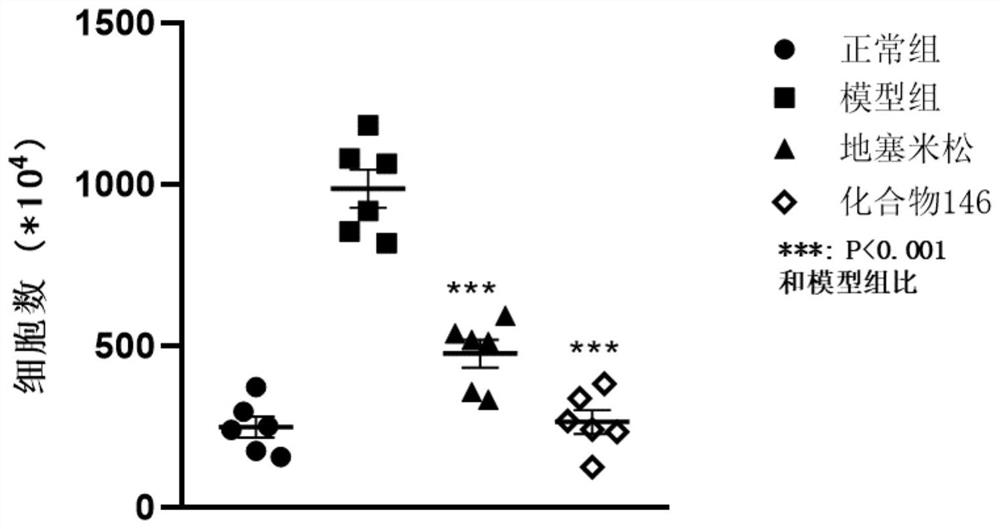
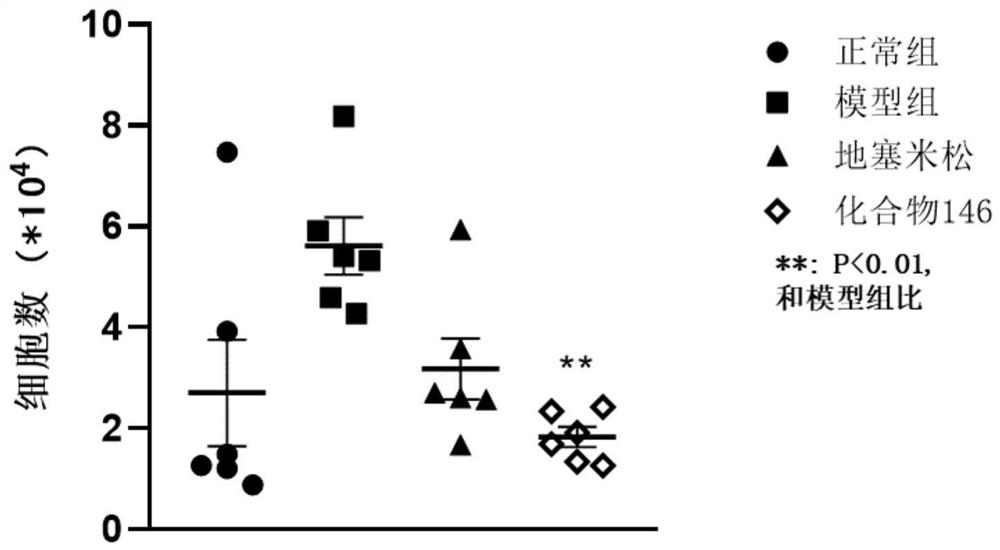
Application of IRAK4 inhibitor in treatment of ALI/ARDS
A technology of inhibitors and uses, applied in the field of medical use, can solve problems such as no IRAK4 inhibitors yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1365] Example 1.N-[2-(3-hydroxyl-3-methylbutyl)-6-(2-hydroxypropan-2-yl)-2H-indazol-5-yl]-6-(trifluoro Preparation of methyl) pyridine-2-amide (146) (synthesized with reference to the preparation method of Example 11 of patent CN107406416)
[1366]
[1367] Step 1: Preparation of methyl 5-nitro-1H-indazole-6carboxylate (Int-2)
[1368]
[1369] In a three-necked flask, dissolve 4.60g (26.1mmol) of 1H-indazole-6-carboxylic acid methyl ester in 120ml of sulfuric acid (96%), and cool to minus 15°C. Cooled nitrating acid (10 ml 96% sulfuric acid in 5 ml 65% nitric acid) was added dropwise. After the dropwise addition, the reaction solution continued to stir for 1 h (internal temperature -13° C.). The reaction solution was added to ice, the precipitate was filtered out with suction, washed with water, and dried in a drying oven at 50°C at low temperature. 5.10 g of the target compound was obtained.
[1370] MS(ESI): m / z=222.1[M+H] + .
[1371] Step 2: Preparation of 5-...
Embodiment 2
[1387] Example 2. (R)-N-(5-(3-hydroxypyrrolidin-1-yl)-2-morpholinooxazolo[4,5-b]pyridin-6-yl)-2-( Preparation of 2-methylpyridin-4-yl)oxazole-4-carboxamide (41)
[1388]
[1389] Step 1: Preparation of 6-chloro-2-nitropyridin-3-ol (Int-7)
[1390]
[1391] 11 g (85.27 mmol) of 6-chloropyridin-3-ol was dissolved in 50 ml of sulfuric acid and cooled to 0°C in an ice-salt bath, and 10 ml of nitric acid (65%) solution was slowly added dropwise while maintaining the reaction temperature below 10°C. After the dropwise addition, continue to stir and react for 3 hours, then pour into 400ml of ice water, stir for 30 minutes, filter, wash the filter cake with water three times, collect and dry the filter cake to obtain 10.43 g of the target compound.
[1392] MS(ESI): m / z=175.1[M-H] + .
[1393] Step 2: Preparation of 2-amino-6-chloropyridin-3-ol (Int-8)
[1394]
[1395] Dissolve 10.43g (0.06mol) of 6-chloro-2-nitropyridin-3-ol in 100ml of ethanol, then add 33.6g (0.6mol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com