Preparation method of anti-adhesion membrane for preventing and treating myophagus adhesion and anti-adhesion membrane
A technology of anti-adhesion membrane and amniotic membrane, which is applied in the field of medical materials, can solve the problems of immunogenicity, fresh amniotic membrane is not suitable for storage, transportation, and clinical difficulties, so as to reduce adhesion, promote endogenous healing of tendon, and reduce adhesion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
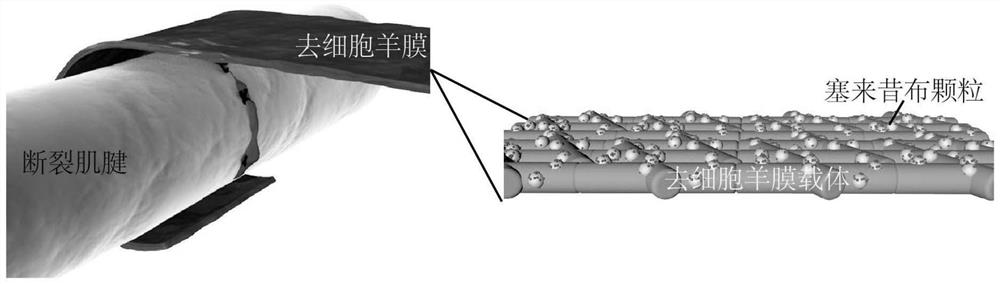
[0030] A method for preparing an anti-adhesion film for preventing and treating sarcolemma,
[0031] S1. Acquisition of fresh amniotic membrane: Fresh placental tissue was bluntly separated between amniotic membrane and chorion to obtain smooth, translucent fresh amniotic membrane, soaked in balanced salt solution containing penicillin and streptomycin, and stored in a refrigerator at 4°C until use;
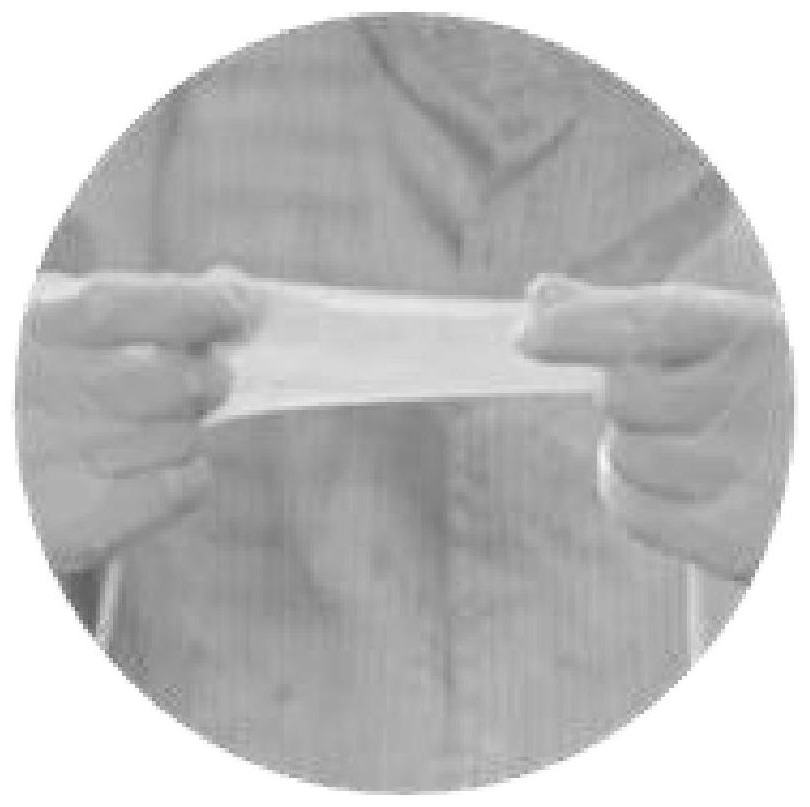
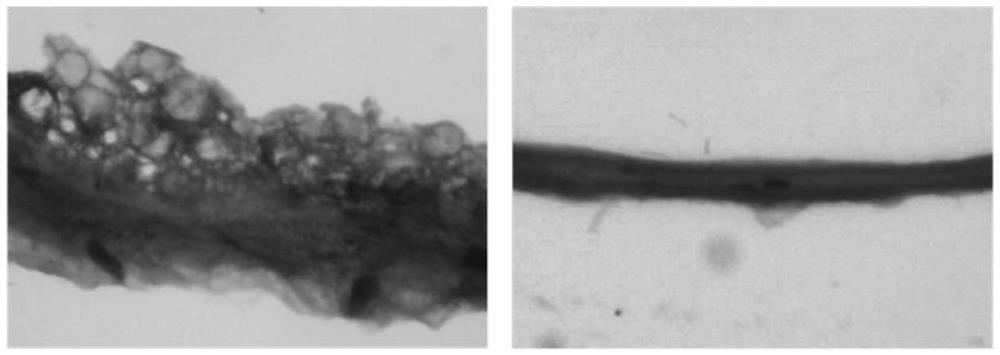
[0032] S2, preparation of decellularized amniotic membrane: fresh human amniotic membrane was rinsed with sucrose phosphate synthase (SPS) solution and cross-linked with glutaraldehyde, and the cross-linked amniotic membrane was shaken in 0.5% sodium dodecyl sulfate (SDS) 24 hours, digested with trypsin for 4 hours, rinsed thoroughly, freeze-dried, subpackaged, sterilized with ethylene oxide, cut into 1.0cm×0.5cm size for later use;
[0033] S3, loading celecoxib: accurately weigh 100mg celecoxib, grind the solid particles, completely dissolve the powder in 10mL absolute ethanol...
Embodiment 2
[0036] An anti-adhesion membrane for preventing and treating sarcolemma, comprising decellularized amniotic membrane on which several celecoxib particles are attached, and the size of the decellularized amniotic membrane is 1.0 cm×0.5 cm.
[0037] The decellularized amniotic membrane contains a variety of bioactive substances that can effectively promote the endogenous healing of the tendon, and celecoxib can reduce inflammation and hinder the adhesion caused by exogenous healing. The combination of the two improves the therapeutic effect of the anti-adhesion membrane and reduces the postoperative recovery time.
Embodiment 3
[0039] Drug sustained release test of celecoxib:
[0040] Accurately weigh 100 mg of celecoxib, grind the solid particles, and completely dissolve the powder in 10 mL of absolute ethanol to prepare a celecoxib mother solution with a concentration of 10 mg / mL. Then it was dissolved in PBS to prepare a series of standard solutions of celecoxib with known concentrations ranging from 0 to 0.1 mg / mL. At 249nm, the absorbance of the celecoxib standard solution with known concentration above was measured with a UV-Vis spectrophotometer (UV-2550, Shimadzu, Japan). Furthermore, the concentration (x) of celecoxib is used as the abscissa, and the absorbance (y) is used as the ordinate to map, so that the solution standard curve equation of celecoxib is: y=1.65x+0.0343, and its correlation The coefficient is r2=0.9998.
[0041] The PCL / AM nanofiber composite films with celecoxib content of 5%, 8%, and 10% were cut into sheets of 20mm×20mm, and accurately weighed. The weight was about 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com