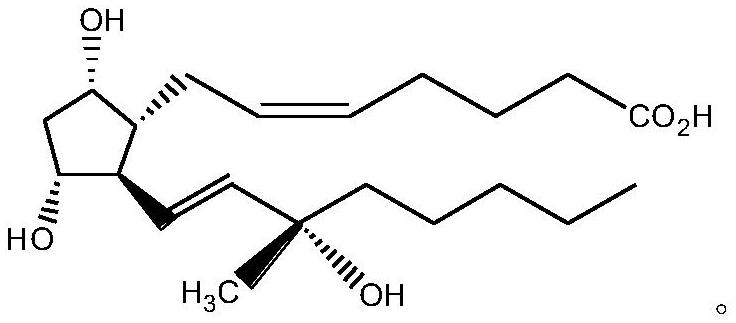
A kind of preparation method of carboprost
A technology of carboprost and esterification, which is applied in the field of preparation of carboprost, can solve the problems of complex post-processing and low combined yield, and achieve the effects of simple post-processing, low production cost, and reduced separation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
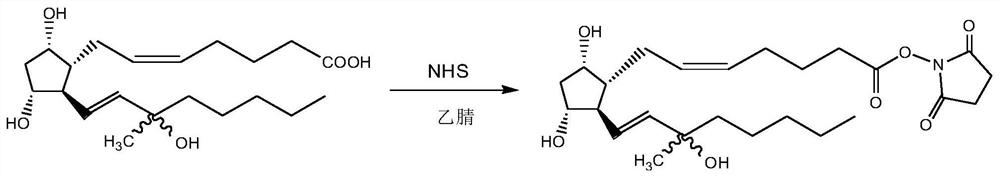
[0028] 1) Crude Carboprost is combined with NHS:
[0029]
[0030] Add 150 mL of acetonitrile to a four-necked flask, add 10.00 g of crude carboprost (the carboprost content was 52.71% detected by HPLC) into 150 mL of acetonitrile, and then add 4.39 g of N,N'-carbonyldiimidazole, N 2 After replacement, stand at room temperature for 2h.
[0031] 4.68g of N-hydroxysuccinimide was added dropwise to a four-necked flask, kept at room temperature for 2h after the drop was completed, HPLC tracking showed that the reaction of the raw materials was complete and the reaction solution was concentrated under reduced pressure to obtain 16.99g of pale yellow amorphous; 1 H NMR (500 MHz, Chloroform-d): δ5.71(t,1H), 5.69(m,1H), 5.46-5.44(m,2H), 3.29(t,1H), 3.21(m,1H), 2.76-2.73(m, 4H), 2.26-2.23(m, 3H), 2.15-2.11(t, 3H), 1.98-1.96(t, 2H), 1.92-1.90(t, 2H), 1.81(t, 2H) ), 1.65(d, 1H), 1.60(m, 2H), 1.44-1.41(m, 5H), 1.33-1.29(m, 6H), 0.96(t, 3H).
[0032] 2) Preparation of carboprost este...
Embodiment 2
[0039] 1) Crude Carboprost is combined with NHS:
[0040]
[0041] Add 150mL DMF to a four-necked flask, add 10.00g crude carboprost (the carboprost content was 51.54% detected by HPLC) into 150mL DMF, and then add 4.39g N,N'-carbonyldiimidazole, N 2 After replacement, stand at room temperature for 2h.
[0042] 4.68g of N-hydroxysuccinimide was added dropwise to a four-necked flask, and kept at room temperature for 2h after dropping. HPLC tracking showed that the reaction of the raw materials was completed, and the reaction solution was concentrated under reduced pressure to obtain 16.78g of pale yellow amorphous.
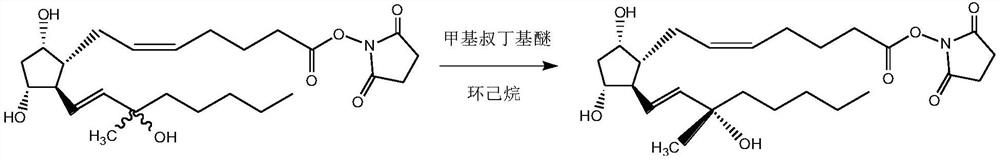
[0043] 2) Preparation of carboprost ester compound:
[0044]
[0045] Into the four-necked flask, add all the light yellow amorphous obtained in the previous step, then add 100 mL of methyl tert-butyl ether and 10 mL of cyclohexane, heat up to 40°C while stirring, keep warm for 2 hours, then drop to room temperature, and let stand for 2 hours , a light yell...
Embodiment 3
[0050] 1) Crude Carboprost is combined with NHS:
[0051]
[0052] In a four-necked flask, add 150 mL of tetrahydrofuran, add 10.00 g of crude carboprost (the carboprost content was 53.55% detected by HPLC) into 150 mL of tetrahydrofuran, and then add 4.39 g of N,N'-carbonyldiimidazole, N 2After replacement, stand at room temperature for 2h.
[0053] 4.68g of N-hydroxysuccinimide was added dropwise to a four-necked flask, and kept at room temperature for 2h after dropping. HPLC tracking showed that the reaction of the raw materials was complete, and the reaction solution was concentrated under reduced pressure to obtain 17.02g of pale yellow amorphous.
[0054] 2) Preparation of carboprost ester compound:
[0055]
[0056] Into the four-necked flask, add all the pale yellow amorphous obtained in the previous step, then add 100 mL of methyl tert-butyl ether and 10 mL of methyl cyclohexane, and heat up to 30°C while stirring, keep the temperature for 2 hours and then drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com