Application of leontopodium leontopodioides to preparation of medicine for treating pulmonary fibrosis
A technology of pulmonary fibrosis and edelweiss, which is applied in the field of edelweiss for the preparation of medicines for treating pulmonary fibrosis, and can solve problems such as no edelweiss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0043] Crush the edelweiss medicinal material and pass through No. 2 sieve, soak 100g of the sieved edelweiss medicinal material powder in water, heat and reflux for extraction 3 times; each time the amount of water is 1000mL, soak for 5h each time, and extract for 4h by heating and reflux each time ; The combined extracts were filtered and concentrated under reduced pressure to obtain 19.8g edelweiss extract. Put it in a 4°C refrigerator and refrigerate it. It can be diluted and dissolved with water when used.
experiment example 1
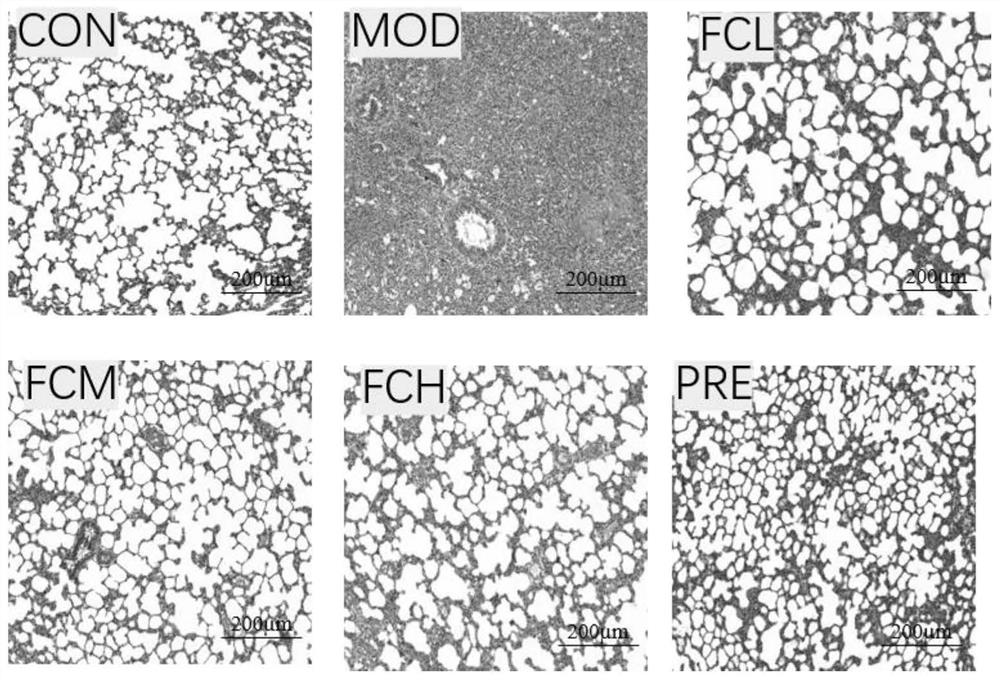
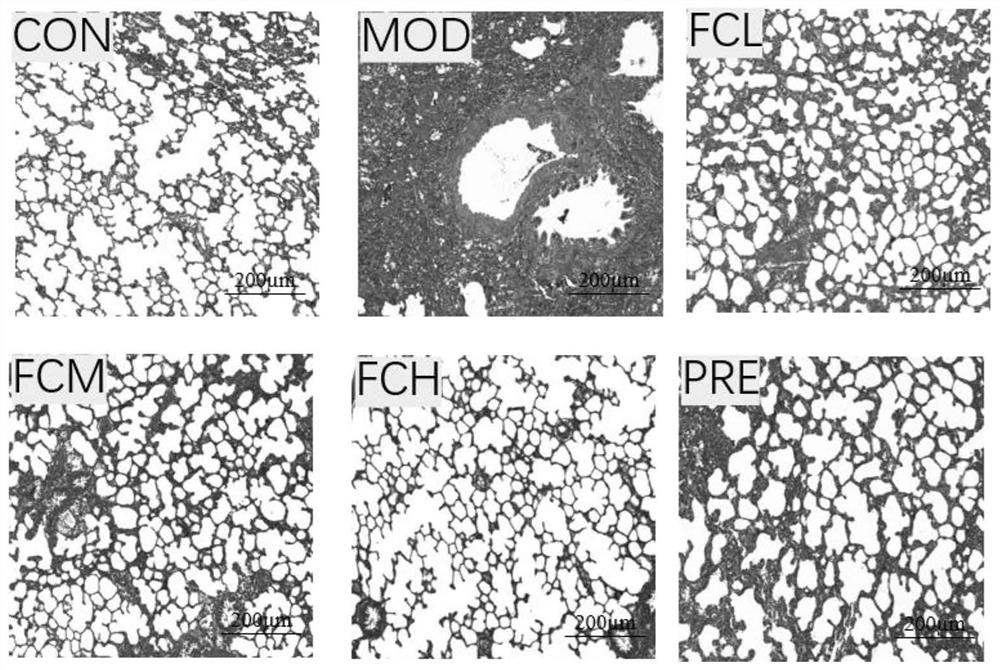
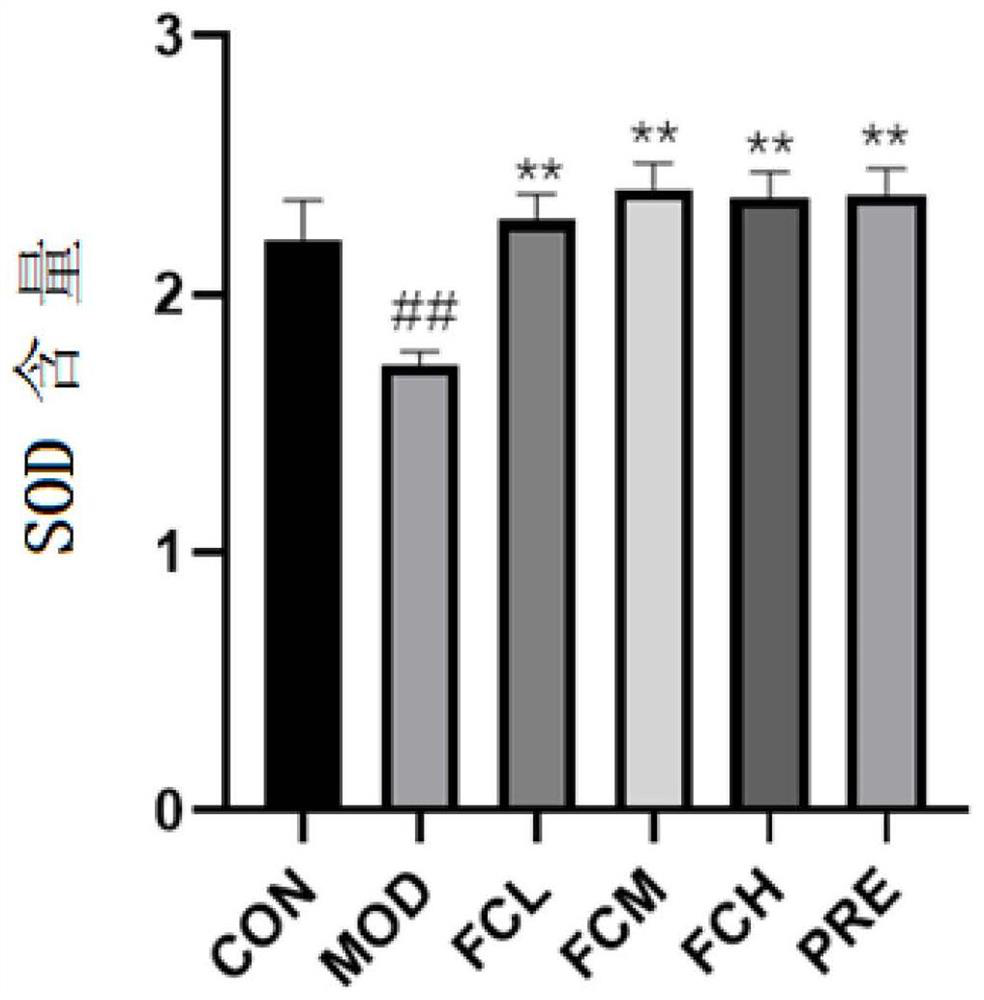
[0045] 1. Animal modeling and experimental methods
[0046] Healthy SD rats, weighing 300-350 g, were used as animals, purchased from Changchun Yisi Experimental Animal Technology Co., Ltd.
[0047] Rats were randomly divided into normal control group, bleomycin model control group (model control group), edelweiss administration group (edelweiss low-dose group, edelweiss middle-dose group, edelweiss high-dose group, edelweiss given The edelweiss extract used in the medicine group is the edelweiss extract prepared in Preparation Example 1) and the prednisone hydrochloride positive drug group (5mg / kg), a total of 6 groups, 10 in each group. Each group was intragastrically administered the corresponding test drug, once a day, until sacrificed, and the model control group and normal control group were intragastrically administered distilled water. Except for the blank group, the other groups established rat pulmonary fibrosis models by injecting bleomycin 5 mg / kg into the trachea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com