Diaminopimelate dehydrogenase mutant and application thereof
A technology of diaminopimelate dehydrogenase and mutants, which is applied in the field of genetic engineering, can solve problems such as the transformation of coenzyme preference in the absence of diaminopimelate dehydrogenase, and achieve low price and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Construction of expression plasmid pET-28a-ddh
[0027] It is derived from C. glutamicum XQ-5 diaminopimelic acid dehydrogenated DAPDH, which is connected to the pET-28a plasmid through restriction enzyme sites EcoRI and XholI.
Embodiment 2
[0028] Example 2: Transforming the plasmid into E.coli BL21(DE3) for expression
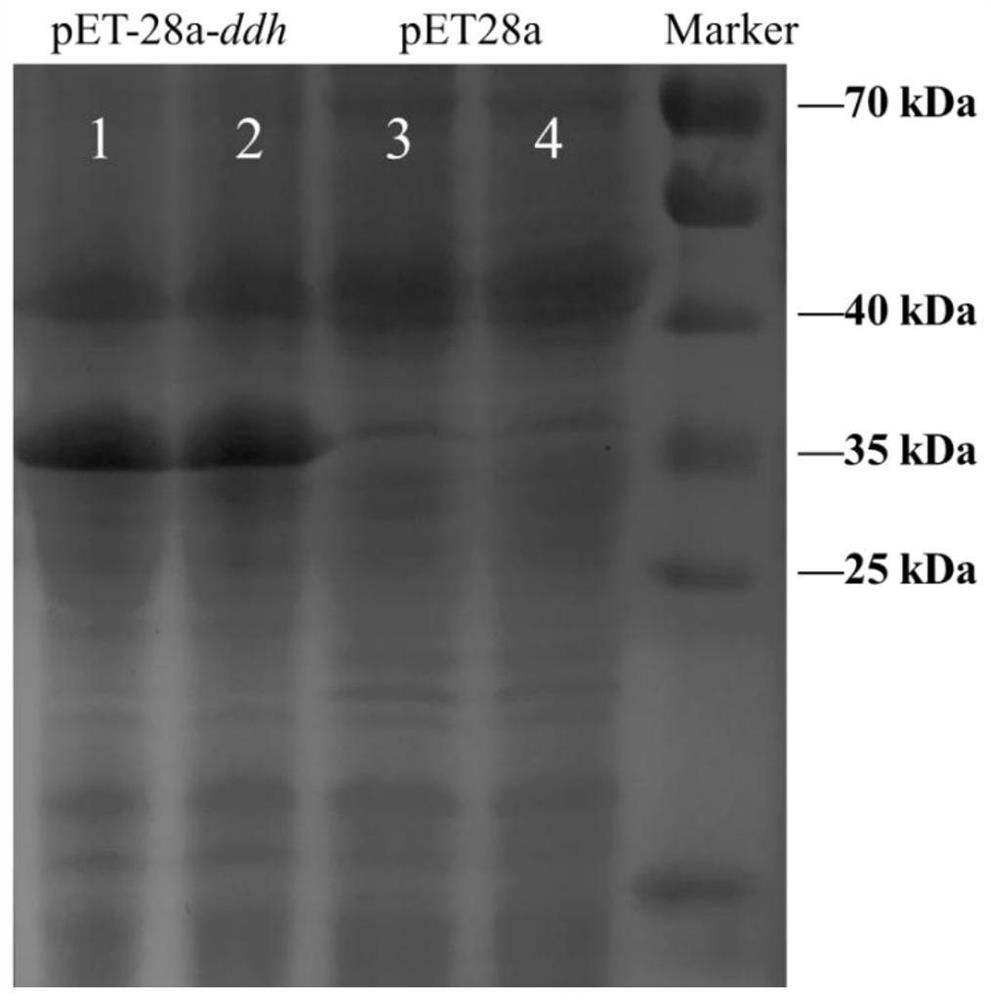
[0029] The recombinant expression plasmid pET-28a-ddh was transformed into E.coli BL21(DE3), and the recombinant expression strain was screened by culturing on LB+Kan solid medium at 37°C. The starting strain and the recombinant strain were first inoculated into LB 10ml liquid vials for primary seed liquid culture for 10-12 hours, then transferred to liquid TB medium with 2% inoculation amount, and cultivated to OD in a shaker at 37°C 600 Between 0.5 and 0.6, a final concentration of 0.5 mM IPTG was added, and the expression was induced at 16° C. for 20 h. Collect the cells after expression, wash twice with PBS buffer (pH 7.4), suspend the cells and control them at the same OD 600 , and then break the bacteria with an ultrasonic breaker, and centrifuge to get the supernatant to obtain the crude enzyme solution. Such as figure 1 , SDS-PAGE was carried out on the crude enzyme solution, and it wa...
Embodiment 3
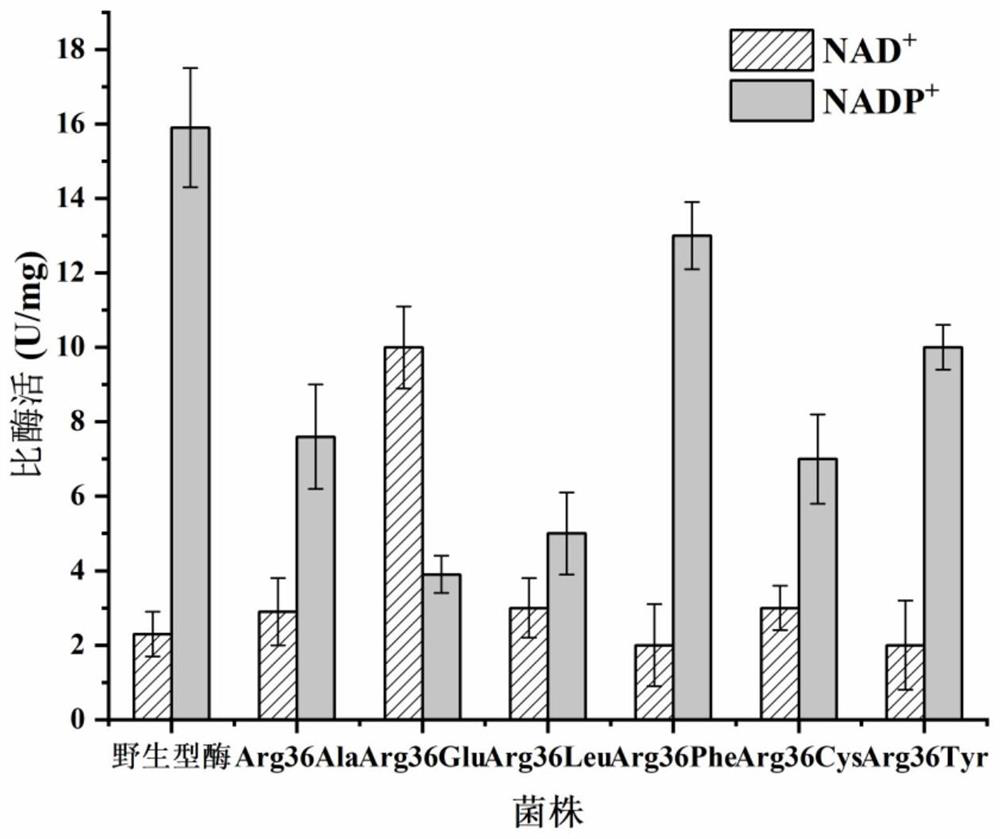
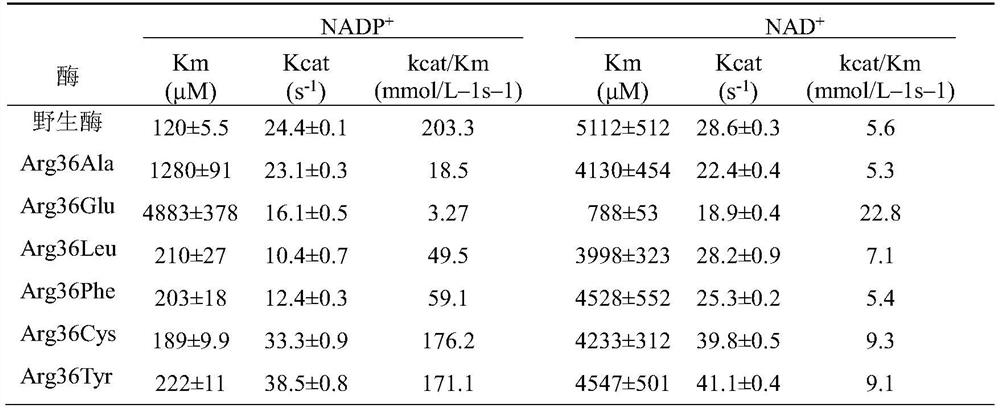
[0030] Embodiment 3: Transform the mutant plasmid into E.coli BL21 (DE3) and measure the kinetic parameters of enzyme activity and enzyme after mutation
[0031] Primers were designed to mutate the 36-arginine of the enzyme DAPDH. Mutations (Arg36Ala, Arg36Glu, Arg36Leu, Arg36Phe, Arg36Cys, Arg36Tyr) were performed using pET-28a-ddh as a template through a point mutation kit. The obtained plasmid containing the mutated gene was transformed into E. coli BL21(DE3). The recombinant strain E.coli BL21(DE3)pET-28a-ddh Arg36Ala Inoculate them in LB 10ml liquid vials for primary seed liquid culture for 10-12 hours, then transfer to liquid TB medium with 2% inoculum amount, and culture in a shaker at 37°C until OD 600 Between 0.5 and 0.6, a final concentration of 0.5 mM IPTG was added, and the expression was induced at 16° C. for 20 h. Collect the cells after expression, wash twice with PBS buffer (pH 7.4), suspend the cells and control them at the same OD 600 , and then break the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com