Method for enriching sulfydryl nitroso protein in serum
A technology of nitrosylation and mercaptonitroso, applied in the field of medicine, can solve the problems of lack of clearing interfering proteins, etc., and achieve the effects of good specificity, high accuracy and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
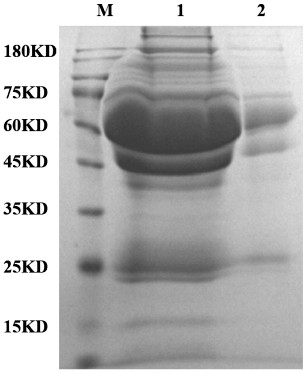
[0051] Collect peripheral blood from fasting humans or animals with vacuum blood collection tubes that do not contain anticoagulants. Let the collected samples stand at 4°C for 6 hours. After the samples are fully coagulated, carefully draw the supernatant into a 1.5mL centrifuge tube and centrifuge at 2000g for 5min to obtain the first supernatant. Transfer the first supernatant to a new 1.5mL centrifuge tube. mL centrifuge tubes to avoid dispersing the precipitate during the aliquoting process, and aliquot the samples at 100 μL / tube and store them at -80°C to avoid repeated freezing and thawing of the samples.
Embodiment 2
[0053] (1) Put the spin column into the collection tube, add 200 μL Protein A agarose and 600 μL glycine-hydrochloric acid buffer Ⅰ to the column in turn, centrifuge at 3000 rpm for 3 minutes, remove the filtrate to wash the spin column, repeat the above operation 4 times, Add 115 μL of the first supernatant obtained in Example 1 and 230 μL of 0.1M sodium phosphate-sodium chloride buffer into a new 1.5mL centrifuge tube, mix well, add the mixture to the cleaned spin column, and place Centrifuge at 3000rpm for 3min on a shaker at 4°C for 1-2h to obtain the first filtrate and the first precipitate.
[0054] (2) Add 2200 μL of glycine-hydrochloric acid buffer II to the spin column containing the first precipitate, and centrifuge at 3000 rpm for 3 minutes to obtain the second filtrate and the second precipitate.
[0055] (3) Combine the first filtrate and the second filtrate, take 500 μL of the mixed filtrate into a new centrifuge tube after pre-cooling for 10 minutes, add 362 μL ...
Embodiment 3
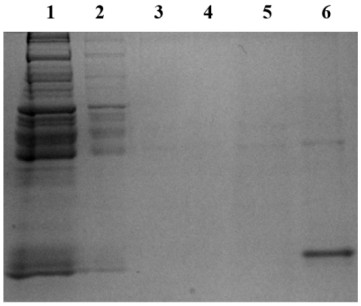
[0058] (1) According to the BCA method (reference: Walker, J.M., The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol, 1994. 32: p. 5-8.) Measure the step (3) of Example 2 to obtain The protein concentration of the protein suspension was adjusted to 1 mg / mL with HENS buffer.
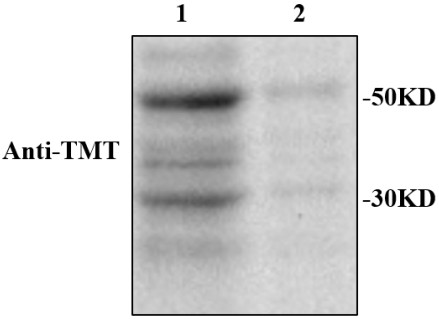
[0059] (2) Take a new centrifuge tube, add 100 μL of the serum with a protein concentration of 1 mg / mL obtained in step (1), and then add 2 μL of 1M MMTS to it, vortex for 1 min, incubate in the dark for 30 min, and place in the tube Add 500 μL of pre-cooled acetone, stand at -20°C for 1 hour, take it out and put it in a refrigerated centrifuge, centrifuge at 1000g at 4°C for 10 minutes, remove acetone, and obtain the fourth filtrate and the fourth precipitate;
[0060] (3) Add 100 μL HENS buffer to the fourth pellet for resuspension, then add 2 μL iodo TMT (Thermo Fisher Scientific Co., Ltd.), vortex to mix, then add 4 μL 1M ascorbic acid, vortex to mix, and incubate for 2 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com