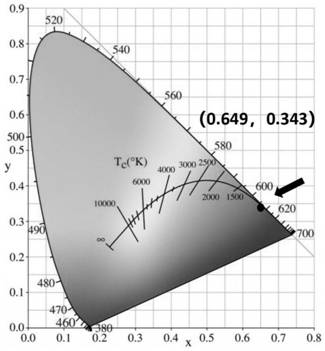
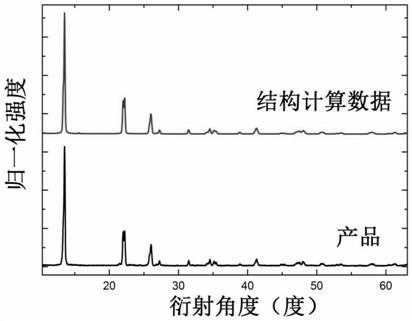
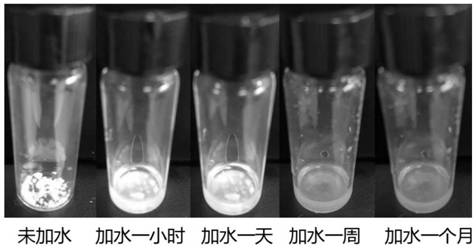
Oleylamine oleic acid modified tetravalent manganese doped fluoride red-light-emitting material and preparation method thereof
A technology of oleylamine oleic acid and tetravalent manganese, which is applied in the field of fluorescent materials, can solve the problems of slow response speed of devices, long fluorescence life, poor stability, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (a) Prepare K by precipitation method 2 MnF 6
[0031] 0.2g KMnO 4 and 4.5g KHF 2 Dissolve in 15 mL HF (48%), stir it for 60 min, then add 0.5 mL hydrogen peroxide dropwise to precipitate a yellow powder K 2 MnF 6 , the precipitate K 2 MnF 6 Filter and clean with acetone and then dry in a vacuum oven for 5 hours;
[0032] (b) Preparation of [N(CH 3 ) 4 ] 2 TiF 6 :Mn 4+ (OA,OC)
[0033] According to the stoichiometric ratio of 1:2, weigh 1.63g of fluorotitanic acid and 1.86g of tetramethylammonium fluoride into a polytetrafluoroethylene beaker, and place the beaker on a magnetic stirring instrument at a speed of 500 rpm. Stir for 30 minutes. Then add oleylamine oleic acid (1mL+0.5mL) according to the stoichiometric ratio of 2:1 and continue to stir for 30 minutes, then dry 0.12g K 2 MnF 6 Dissolved in 10ml HF (48%). The dissolved K 2 MnF6 Slowly add dropwise to the beaker containing fluorotitanic acid and tetramethylammonium fluoride, and continue to st...
Embodiment 2
[0041] (a) Prepare K by precipitation method 2 MnF 6
[0042] 0.2g KMnO 4 and 4.5g KHF 2 Dissolve in 15 mL HF (48%), stir it for 60 min, then add 0.5 mL hydrogen peroxide dropwise to precipitate a yellow powder K 2 MnF 6 , the precipitate K 2 MnF 6 Filter and clean with acetone and then dry in a vacuum oven for 5 hours;
[0043] (b) Preparation of [N(CH 3 ) 4 ] 2 ZrF 6 :Mn 4+ (OA,OC)
[0044] According to the stoichiometric ratio of 1:2, weigh 2.54g of fluorozirconic acid and 1.86g of tetramethylammonium fluoride into a polytetrafluoroethylene beaker, and place the beaker on a magnetic stirring instrument at a speed of 500 rpm. Stir for 30 minutes. Then add oleylamine oleic acid (1mL+0.5mL) according to the stoichiometric ratio of 2:1 and continue to stir for 30 minutes, then dry 0.12g K 2 MnF 6 Dissolved in 10ml HF (48%). The dissolved K 2 MnF 6 Slowly add dropwise to the beaker containing fluozirconic acid and tetramethylammonium fluoride, and continue to ...
Embodiment 3
[0047] (a) Prepare K by precipitation method 2 MnF 6
[0048] 0.2g KMnO 4 and 4.5g KHF 2 Dissolve in 15 mL HF (48%), stir it for 60 min, then add 0.5 mL hydrogen peroxide dropwise to precipitate a yellow powder K 2 MnF 6 , the precipitate K 2 MnF 6 Filter and clean with acetone and then dry in a vacuum oven for 5 hours;
[0049] (b) Preparation of [N(CH 3 ) 4 ] 2 SiF 6 :Mn 4+ (OA,OC)
[0050] According to the stoichiometric ratio of 1:2, weigh 1.91 g of fluorosilicic acid and 1.86 g of tetramethylammonium fluoride in a polytetrafluoroethylene beaker, and place the beaker on a magnetic stirring instrument at a speed of 500 rpm for vigorous stirring 30 minutes. Then add oleylamine oleic acid (1mL+0.5mL) according to the stoichiometric ratio of 2:1 and continue to stir for 30 minutes, then dry 0.12g K 2 MnF 6 Dissolved in 10ml HF (48%). The dissolved K 2 MnF 6 Slowly add dropwise to the beaker containing fluosilicic acid and tetramethylammonium fluoride, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com