Microbial integrity test device and test method for closed sterile barrier system
A sterile barrier and test device technology, applied in enzymology/microbiology devices, methods of supporting/immobilizing microorganisms, biochemical cleaning devices, etc., can solve the problem of unreasonable control of the production quality and cost of packaged products Incapable of quantitative determination, over-design of the seal, etc., to achieve the effect of controlling manufacturing costs, avoiding over-design, and ensuring quality inspection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
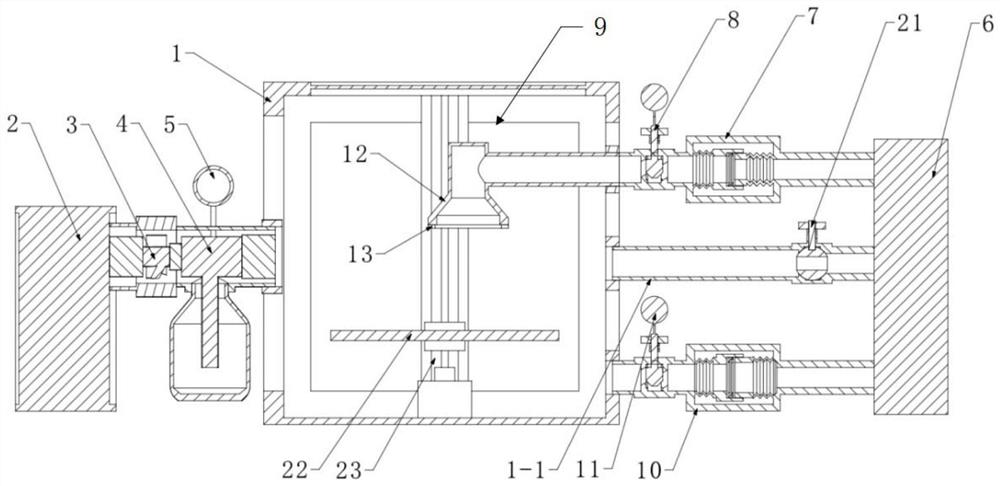
[0058] Such as figure 1 As shown, a closed sterile barrier system microbial integrity test device, including a test box 1, an aerosol generating system and a vacuum pump 6, the right side of the test box 1 is provided with an exhaust pipeline 1-1, the exhaust The pipeline 1-1 is connected with the vacuum pump 6 through the valve 21; the aerosol generating system is connected with an air filter 2, a compressor 3 and a nebulizer 4 sequentially from left to right, and the nebulizer 4 is connected with the test chamber 1, so The sprayer 4 is provided with a flow control valve 5; the test device is also provided with a microbial integrity detection system, and the microbial integrity detection system includes a test environment detection device and a packaging detection device.
[0059] The package detection device includes a package connector 9, a first colony collector 7 positioned on the right side of the test box 1 and a first flow regulating valve 8 positioned on the right sid...
Embodiment 2
[0069] Such as Figure 4 with Figure 5 As shown, different from Embodiment 1, the packaging connector 9 includes a bowl-shaped cover 12 , a vacuum suction cup 14 and a sealing ring 15 .
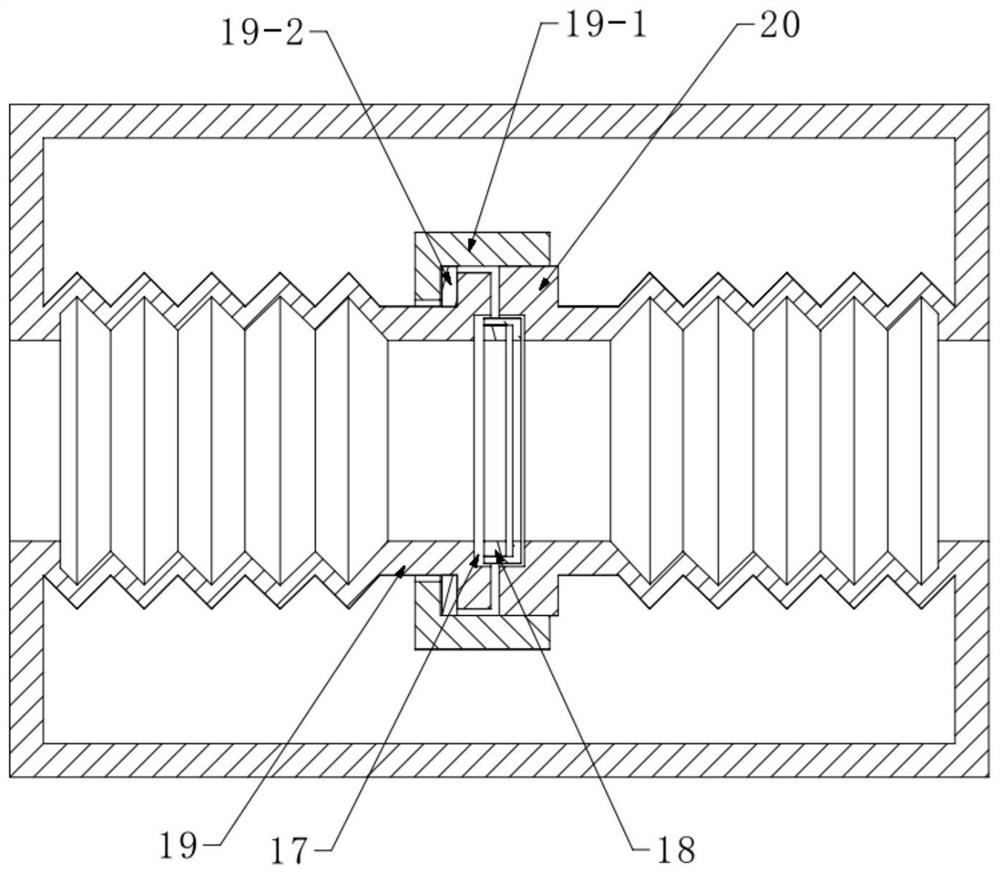
[0070] Described vacuum chuck 14 comprises adsorption ring 14-1, vacuum suction pipe 14-2 and vacuum generator 14-3, and described adsorption ring 14-1 is arranged on the lower end surface of bowl-shaped cover 12, and the adsorption of described adsorption ring 14-1 The cavity is connected to a vacuum generator 14-3 through a vacuum suction pipe 14-2, and the vacuum generator 14-3 is fixedly arranged on the bowl-shaped cover 12. The sealing ring 15 cooperates with the lower end surface of the adsorption ring 14-1.
[0071] In this embodiment, it is necessary to fix the sealing ring 15 and the packaging sample to be tested together, and then cut out an opening in the sealing ring 15, so that the adsorption ring 14-1 and the sealing ring 15 are adsorbed together, so as to ensure that the ins...
Embodiment 3
[0073] Such as Image 6 As shown, on the basis of Embodiment 1, the packaging connector 9 is also provided with an opener 16, and the opener 16 includes a lifting pipe 16-1, a lifting motor 16-2, an electric push rod 16-3, a rotary Motor 16-4 and cutting tool 16-5, vertically arranged in the lifting pipeline 16-1 in the test chamber 1 is connected with the bowl-shaped cover 12, and the described lifting motor 16-2 is fixedly arranged in the lifting pipeline 16-1, so The lifting motor 16-2 is connected with an electric push rod 16-3, the lower end of the electric push rod 16-3 is fixed with a rotating motor 16-4, and the motor shaft of the rotating motor 16-4 is fixedly provided with a cutting tool 16-5 .
[0074] In this embodiment, double-sided sealant is used to seal the packaged sample to be tested with the bowl-shaped cover 12, and then the opener 16 is used to cut out the opening of the packaged sample to be tested in the bowl-shaped cover 12, so that the inside of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com