Anchor sequence identified by T7 phage, DNA vaccine recombinant plasmid and application
A DNA vaccine and anchor sequence technology, applied in the field of genetic engineering technology, can solve problems such as difficulty in popularization, unsatisfactory immune protection efficiency, and sudden reduction in the number of DNA vaccines, so as to reduce the risk of degradation and facilitate operation , using a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
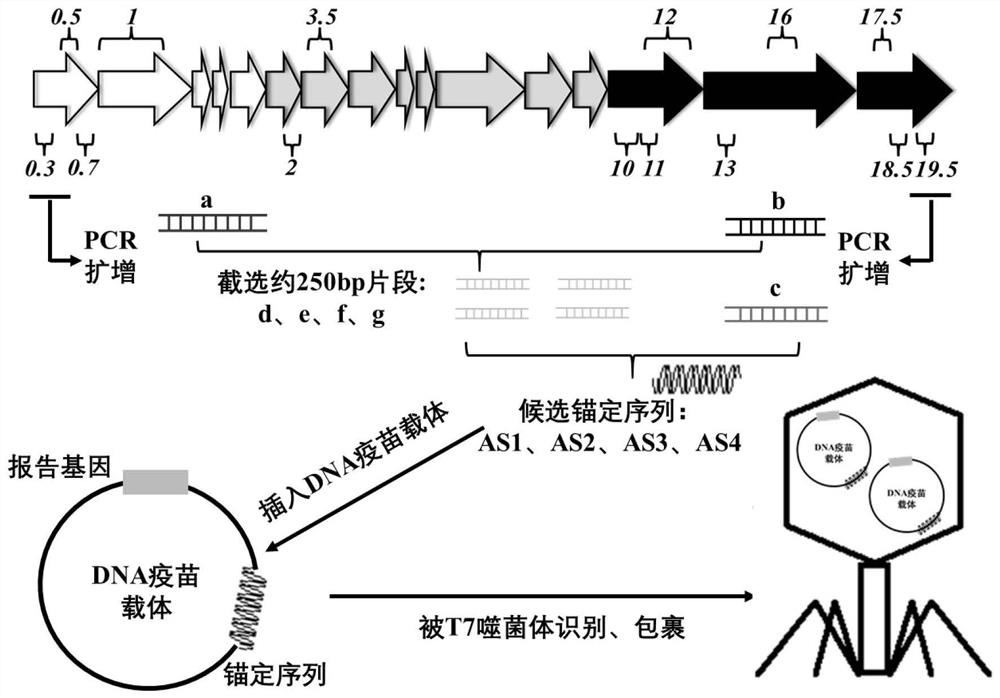
[0029] Example 1 Construction of a recombinant DNA vaccine vector carrying a candidate anchor sequence, the construction route method is as follows figure 1 shown
[0030] 1. PCR amplification of candidate anchor sequences
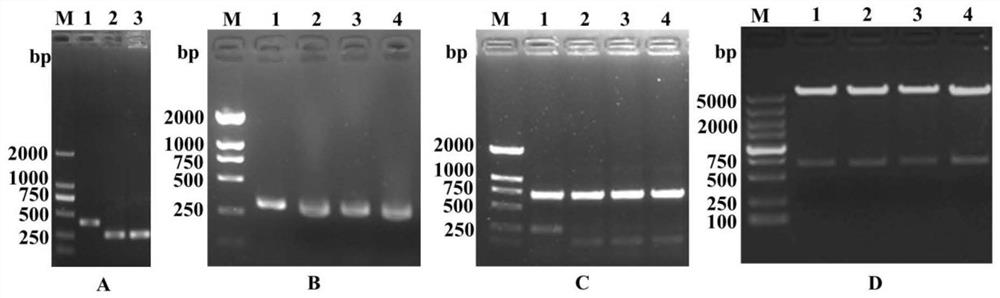
[0031] Using the T7 phage genome as a template, primer F1 / R1 amplifies positions 1 to 276 on the left side of the genome (fragment a), such as figure 2 -Shown in 2 swimming lanes in A; primer F2 / R2 amplifies positions 39681 to 39936 on the right (fragment b), as figure 2 -Shown in 3 swimming lanes in A; Primer F3 / R3 amplifies position 38981 to 39371 on the right side (fragment c), as figure 2 -Shown in lane 1 in A. Since the 5' end of fragment a is complementary to the 3' end of fragment b, fragments a and b can be spliced into a complete sequence in the PCR system. Using the complete sequence as a template, design primers F4 / R4 (corresponding to fragment d), F5 / R5 (corresponding to fragment e), F6 / R6 (corresponding to fragment f) and F7 / R7 (corre...
Embodiment 2T7
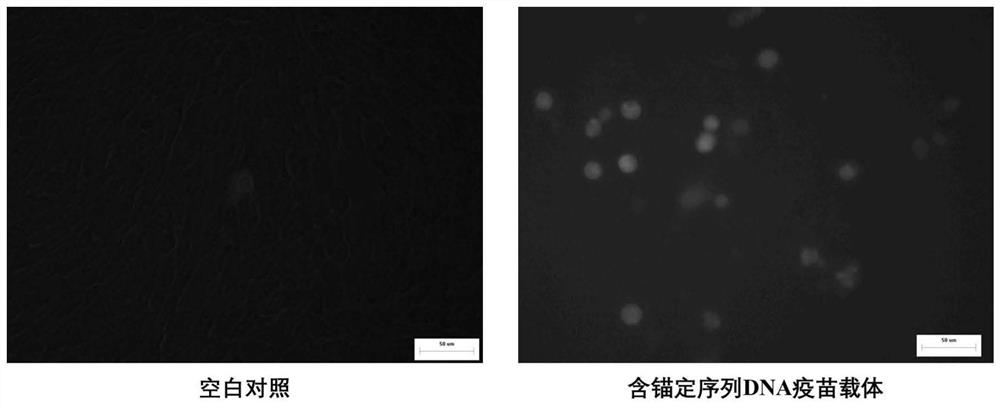
[0052] The protection of embodiment 2T7 phage encapsulation to DNA vaccine carrier
[0053] 1. Small-scale preparation of T7 phage encapsulating DNA vaccine vector
[0054] The recombinant bacteria constructed in Step 2 of Example 1 were cultured in four sections on the ampicillin-resistant plate. Pick a monoclonal colony and inoculate 3 mL of LB culture medium containing ampicillin resistance, and culture overnight on a shaker at 37°C. At the same time, Escherichia coli carrying the DNA vaccine vector (without inserting the anchor sequence) was cultured as a control. On the next day, transfer the cultured two groups of seed solutions to 3 mL of fresh culture solution at a ratio of 1:100, and continue to cultivate to OD 600 nm=1.0, respectively inoculate 5 μL T7 phage seeds (10 10 pfu / mL), continue to cultivate for 2-3 hours until E. coli is completely lysed. At this time, the T7 phage infects the recombinant bacteria carrying the anchor sequence, and the inside of the cap...
Embodiment 3T7
[0060] Efficiency evaluation of embodiment 3T7 phage encapsulating DNA vaccine carrier
[0061] 1. Establishment of DNA vaccine vector fluorescent quantitative PCR method
[0062] Using the recombinant DNA vaccine vector prepared in Step 2 of Example 1 as a template, a fluorescent quantitative PCR method was established by detecting the reporter gene EGFP. First, use Nanodrop to quantify the recombinant DNA vaccine vector (plasmid), and calculate the corresponding relationship between plasmid copy number and quality according to the molecular weight of the recombinant DNA vaccine vector. Adjust the concentration of the DNA vaccine carrier, serially dilute to 1, 10, 100, 1000, 10000, 100000 and 1000000 copies / μL respectively, take 1 μL of the carrier dilution as a template, establish a fluorescent quantitative PCR detection method, and draw a standard curve such as Figure 5 As shown in C, the calculation formula y=-3.257x+38.45 is obtained, which is used to calculate the copy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com