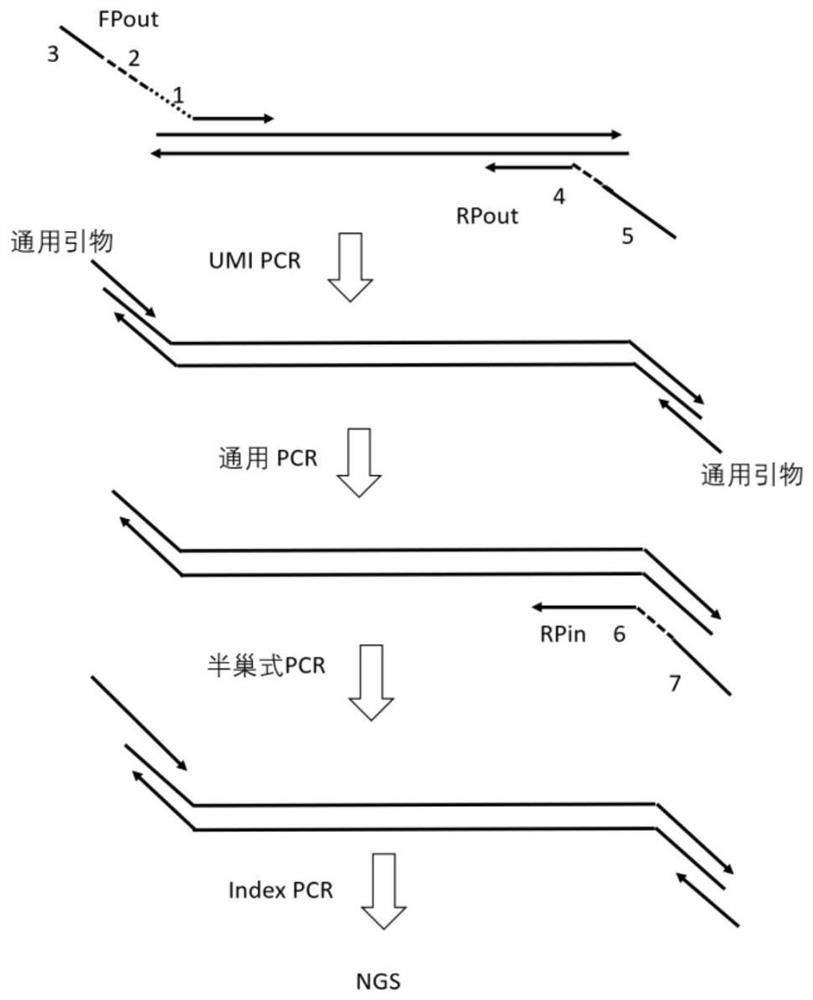
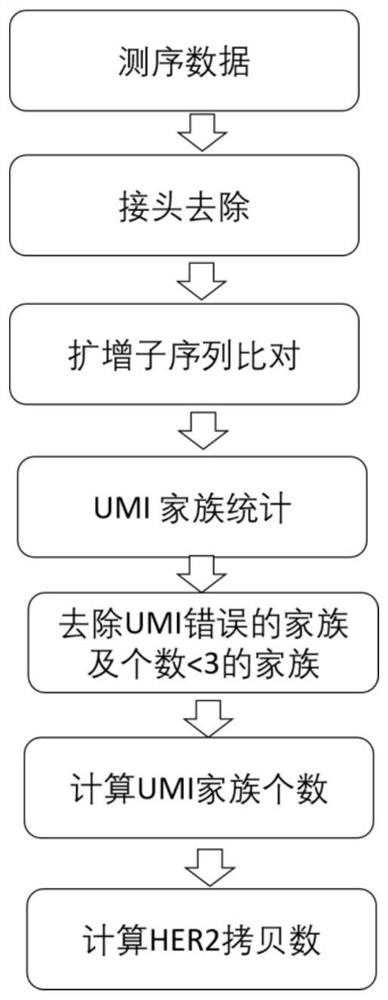
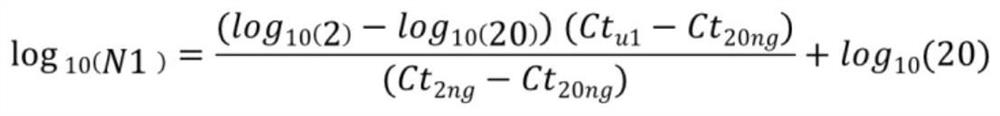Method and kit for detecting human HER2 gene copy number variation
A technology of gene copy number and copy number variation, which is applied in the fields of genomics, biochemical equipment and methods, and microbial measurement/inspection, to achieve the effects of simple process, improved sensitivity, and low sample size requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Detection of HER2 copy number variation in tumor FFPE DNA samples from patients with gastric cancer
[0042] (1) Sample DNA extraction
[0043]FFPE DNA was extracted using Qiagen GeneRead DNA FFPE kit, and the specific operation was performed according to the instructions of the kit. Extract 4 normal HER2-negative samples and 4 FFPE samples from gastric cancer HER2-positive patients. Quantification with Qubit fluorescence.
[0044] (2) qPCR accurately quantifies FFPE and cfDNA by using two pairs of housekeeping gene primers ref1 and ref2 (ref1-fP gcacaacattttgtctccggaaaata, ref1-rP gctccagatgggcagcac; ref2-fPgacaaatgcccagaaatggaactta, ref2-rP ttggcagtctttaagatccatagaaatac) to test samples and two An internal reference uninterrupted gDNA sample (2 μl 1ng / μl and 2 μl 10ng / μl) was amplified to obtain the ct value, and the concentration of the primers was 4 μM. The quantitative formula is:
[0045]
[0046]
[0047] N-avg=Average(N1+N2)
[0048] Nu=10 ...
Embodiment 2
[0072] Example 2: Detection of HER2 copy number variation in tumor peripheral blood cfDNA samples of patients with gastric cancer
[0073] (1) DNA extraction
[0074] cfDNA extraction using Nanke Zhengtu Apostle MiniMax TM Free DNA isolation and enrichment kit, specifically according to the instructions of the kit. Extract 4 normal HER2-negative samples, and extract 4 gastric cancer HER2-positive patients. Quantification with Qubit fluorescence.
[0075] (2) qPCR accurately quantifies FFPE and cfDNA by using two pairs of housekeeping gene primers ref1 and ref2 (ref1-fP gcacaacattttgtctccggaaaata, ref1-rP gctccagatgggcagcac; ref2-fPgacaaatgcccagaaatggaactta, ref2-rP ttggcagtctttaagatccatagaaatac) to test samples and 2 An internal reference uninterrupted gDNA sample (2 μl 1ng / μl and 2 μl 10ng / μl) was amplified to obtain the ct value, and the concentration of the primers was 4 μM. The quantitative formula is:
[0076]
[0077]
[0078] N-avg=Average(N1+N2)
[0079] Nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com