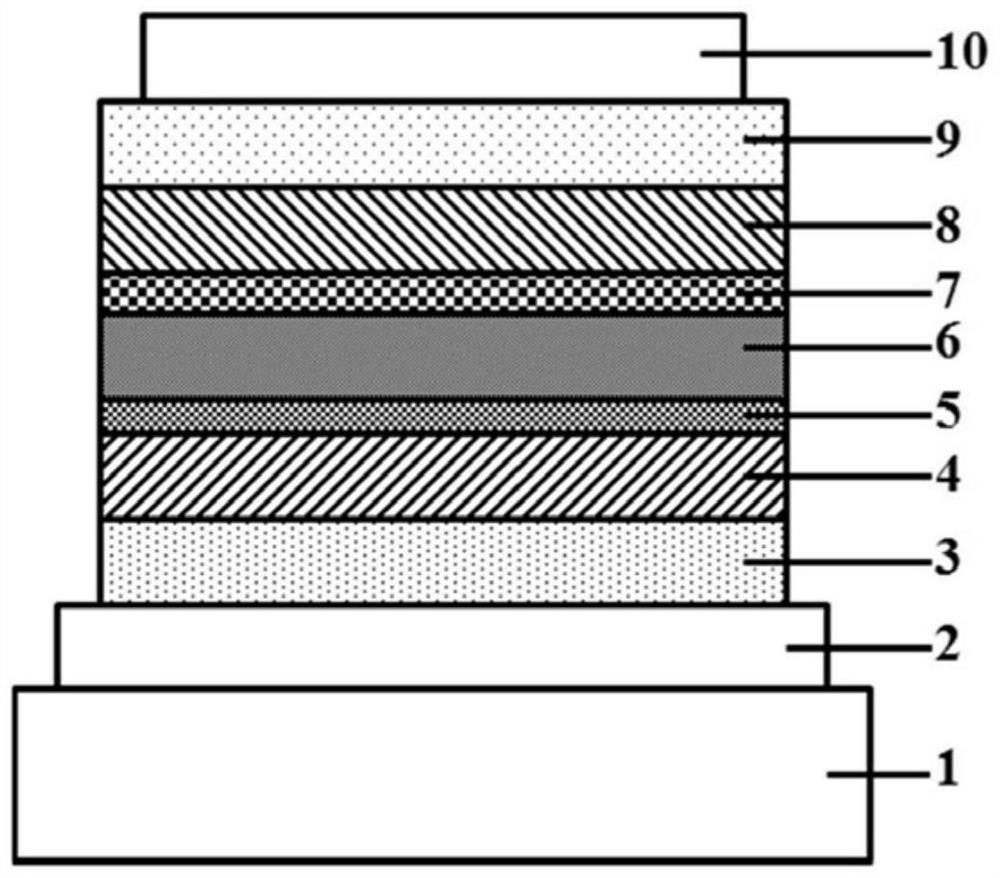
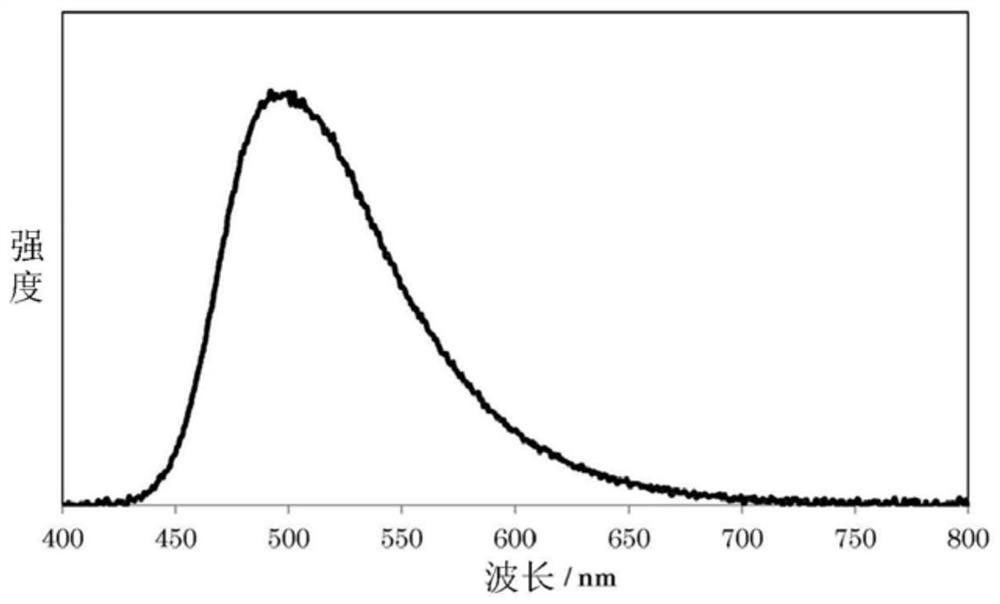
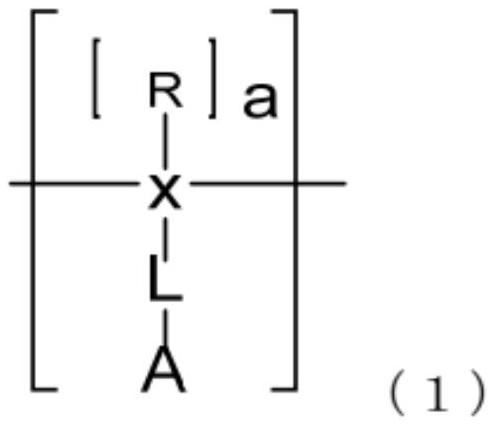
Polymer for organic electroluminescent elements and organic electroluminescent element
A light-emitting element and organic electric field technology, which is applied in the field of polymers for organic electric field light-emitting elements, can solve problems such as insufficient materials, low molecular weight compounds, and difficult lamination and film formation, and achieve high performance, high luminous efficiency, and high driving stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Polymer P-4 was synthesized via Intermediate A, Intermediate B, Intermediate C, Polymerized Intermediate A, Polymerized Intermediate B.
[0242] (Synthesis of Intermediate A)
[0243] [chemical 20]
[0244]
[0245] Under a nitrogen atmosphere, 5.13 g (20.0 mmol) of 5,12-dihydroindolo[3,2-a]carbazole, 2-(2-fluorophenyl)-4,6-diphenyl-1 , 7.21 g (22.0 mmol) of 3,5-triazine, 32.61 g (100.1 mmol) of cesium carbonate, and 100 ml of N-N-dimethylacetamide (dehydrated) were stirred. Then, it heated to 140 degreeC, and stirred for 24 hours. After cooling the reaction solution to room temperature, inorganic substances were separated by filtration. The filtrate was dried under reduced pressure and purified by column chromatography to obtain 8.1 g (14.4 mmol, yield 71.8%) of intermediate A as a pale yellow powder.
[0246] (Synthesis of Intermediate B)
[0247] [chem 21]
[0248]
[0249] Under nitrogen atmosphere, add intermediate A 2.00g (3.5mmol), 3,5-dibromoiodobenzen...
Embodiment 2
[0262] Polymer P-13 was synthesized via Intermediate D, Intermediate E, Intermediate F, Intermediate G, Intermediate H and Polymerized Intermediate C, Polymerized Intermediate D.
[0263] (Synthesis of Intermediate D)
[0264] [chem 24]
[0265]
[0266] Under nitrogen atmosphere, add 9-phenyl-9H, 9'H-3,3'-bicarbazole 8.15g (20.0mmol), 3,5-dibromoiodobenzene 9.38g (25.9mmol), copper iodide 0.11 g (0.6 mmol), 21.17 g (99.8 mmol) of tripotassium phosphate, 0.91 g (8.0 mmol) of trans-1,2-cyclohexanediamine, and 80 ml of 1,4-dioxane were stirred. Then, it heated to 120 degreeC, and stirred for 24 hours. After cooling the reaction solution to room temperature, inorganic substances were separated by filtration. The filtrate was dried under reduced pressure and purified by column chromatography to obtain 9.60 g (14.9 mmol, yield 74.9%) of intermediate D as a light yellow powder.
[0267] (Synthesis of Intermediate E)
[0268] [chem 25]
[0269]
[0270] Under nitrogen atm...
Embodiment 3~5、 comparative example 1、2
[0290] The following polymers were synthesized by a synthetic method similar to that described.
[0291] [chem 30]
[0292]
[0293]ΔE (eV) calculated from the following formula was calculated for the polymers obtained in the above examples, and 2-5 and 2-6 synthesized for comparison.
[0294] ΔE(A)=S1(A)-T1(A)
[0295] ΔE(B)=S1(B)-T1(B)
[0296] The calculation results are shown in Table 1, and the GPC measurement results and solubility evaluation results are shown in Table 2. The case where ΔE satisfies 0.5 eV or less is defined as ΔE(A), and the case where ΔE exceeds 0.5 eV is defined as ΔE(B).
[0297] [Table 1]
[0298] polymer ΔE(A) ΔE(B) Example 1 P-4 0.03 - Example 2 P-13 0.19 0.73 Example 3 P-1 0.20 - Example 4 P-7 0.17 0.73 Example 5 P-12 0.03 - Comparative example 1 2-5 - 0.73 / 0.72 Comparative example 2 2-6 - 0.51
[0299] [Table 2]
[0300] polymer mw mn Mw / Mn S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com