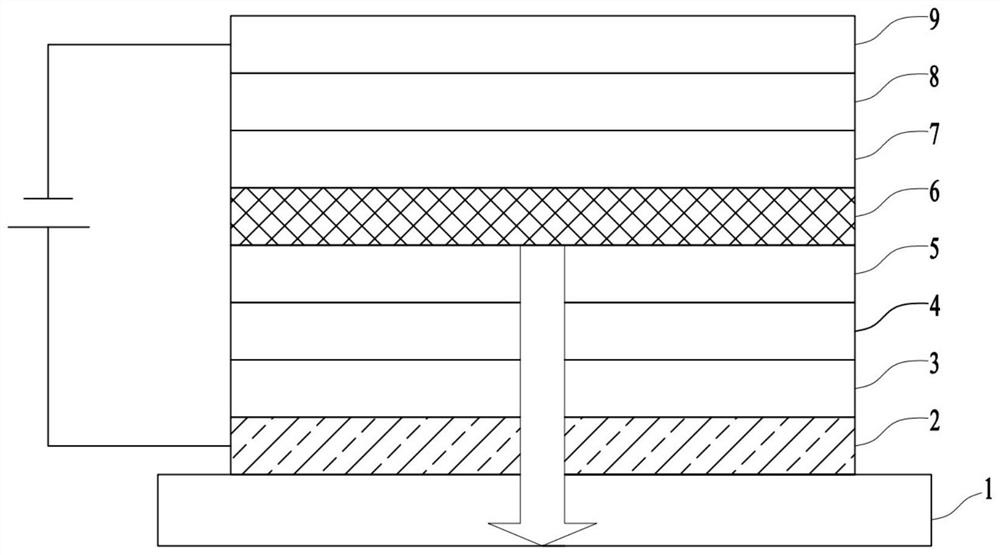
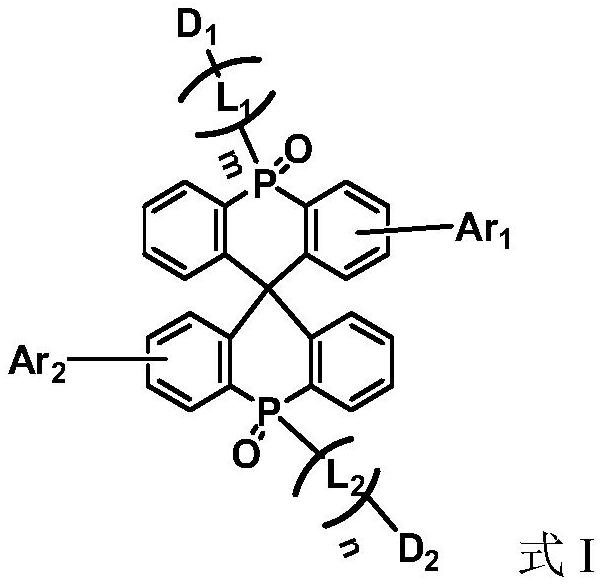
Compound, display panel and display device
A compound, unsubstituted technology, applied in the field of organic electroluminescent materials, can solve the problems of concentration quenching, performance degradation of OLED display devices, triplet-triplet annihilation, etc., and achieves reduced intermolecular stacking and good thermodynamic stability. , the effect of increasing the triplet energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Synthesis of Compound M003
[0128]
[0129] Under nitrogen protection, compound X010 (10.5 mmol) was weighed and added to a 500 mL two-necked flask, and 80 mL of dry anhydrous tetrahydrofuran was added to dissolve X010. NaH (stored in 60% oil, 11.0 mmol) was repeatedly washed with n-hexane three times, added to a two-necked flask in batches, and stirred for 1 h. Then X009 (5 mmol) was added to the two-necked flask, reacted at room temperature, and stirred overnight. Quench the reaction with methanol and water, extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography and finally by sublimation to give solid X011 (3.1 mmol, 62%).
[0130] Obtained by liquid chromatography-mass spectrometry analysis:
[0131] MALDI-TOF MS: m / z calcd for C6...
Embodiment 2
[0141] Synthesis of Compound M002
[0142]
[0143] Under nitrogen protection, compound X010 (10.5 mmol) was weighed and added to a 500 mL two-necked flask, and 80 mL of dry anhydrous tetrahydrofuran was added to dissolve X010. NaH (stored in 60% oil, 11.0 mmol) was repeatedly washed with n-hexane three times, added to a two-necked flask in batches, and stirred for 1 h. Then X006 (5 mmol) was added to the two-necked flask, reacted at room temperature, and stirred overnight. Quench the reaction with methanol and water, extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel chromatography, and finally purified by sublimation to obtain solid X012 (yield 68%).
[0144] Obtained by liquid chromatography-mass spectrometry analysis:
[0145] MALDI-TOF MS: m / z calcd for C...
Embodiment 3
[0155] Synthesis of Compound M001
[0156]
[0157] Under nitrogen protection, compound X010 (10.5 mmol) was weighed and added to a 500 mL two-necked flask, and 80 mL of dry anhydrous tetrahydrofuran was added to dissolve X010. NaH (stored in 60% oil, 11.0 mmol) was repeatedly washed with n-hexane three times, added to a two-necked flask in batches, and stirred for 1 h. Then X003 (5 mmol) was added to the two-necked flask, reacted at room temperature, and stirred overnight. The reaction was quenched with methanol and water, extracted with dichloromethane, and the organic phase was collected and dried over anhydrous Na2SO4. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography, and finally purified by sublimation to obtain solid X013 (yield 74%).
[0158] Obtained by liquid chromatography-mass spectrometry analysis:
[0159] MALDI-TOF MS: m / z calcd ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com